Neuroscience - Blog Posts

What Happens in the Brain During Unconsciousness?
Researchers are shining a light on the darkness of the unconscious brain. Three new studies add to the body of knowledge.
When patients undergo major surgery, they’re often put under anesthesia to allow the brain to be in an unconscious state.
But what’s happening in the brain during that time?
Three Michigan Medicine researchers are authors on three new articles from the Center for Consciousness Science exploring this question — specifically how brain networks fragment in association with a variety of unconsciousness states.
“These studies come from a long-standing hypothesis my colleagues and I have had regarding the essential characteristic of why we are conscious and how we become unconscious, based on patterns of information transfer in the brain,” says George A. Mashour, M.D., Ph.D., professor of anesthesiology, director of the Center for Consciousness Science and associate dean for clinical and translational research at the University of Michigan Medical School.
In the studies, the team not only explores how the brain networks fragment, but also how better to measure what is happening.
“We’ve been working for a decade to understand in a more refined way how the spatial and temporal aspects of brain function break down during unconsciousness, how we can measure that breakdown and the implications for information processing,” says UnCheol Lee, Ph.D., physicist, assistant professor of anesthesiology and associate director of the Center for Consciousness Science.
Examining different aspects of unconsciousness
The basis for the three studies, as well as other work from the Center for Consciousness Science, comes from a theory Mashour produced during his residency.
“I published a theoretical article when I was a resident in anesthesiology suggesting that anesthesia doesn’t work by turning the brain off, per se, but rather by isolating processes in certain areas of the brain,” Mashour says. “Instead of seeing a highly connected brain network, anesthesia results in an array of islands with isolated cognition and processing. We have taken this thought, as well as the work of others, and built upon it with our research.”
In the study in the Journal of Neuroscience, the team analyzed different areas of the brain during sedation, surgical anesthesia and a vegetative state.
“It’s often suggested that different areas of the brain that typically talk to one another get out of sync during unconsciousness,” says Anthony Hudetz, Ph.D., professor of anesthesiology, scientific director of the Center for Consciousness Science and senior author on the study. “We showed in the early stages of sedation, the information processing timeline gets much longer and local areas of the brain become more tightly connected within themselves. That tightening might lead to the inability to connect with distant areas.”
In the Frontiers in Human Neuroscience study, the team delved into how the brain integrates information and how it can be measured in the real world.
“We took a very complex computational task of measuring information integration in the brain and broke it down into a more manageable task,” says Lee, senior author on the study. “We demonstrated that as the brain gets more modular and has more local conversations, the measure of information integration starts to decrease. Essentially, we looked at how the brain network fragmentation was taking place and how to measure that fragmentation, which gives us the sense of why we lose consciousness.”
Finally, the latest article, in Trends in Neurosciences, aimed to take the team’s previous studies and other work on the subject of unconsciousness and put together a fuller picture.
“We examined unconsciousness across three different conditions: physiological, pharmacological and pathological,” says Mashour, lead author on the study. “We found that during unconsciousness, disrupted connectivity in the brain and greater modularity are creating an environment that is inhospitable to the kind of efficient information transfer that is required for consciousness.”
How these studies can help patients
The team members at the Center for Consciousness Science note that all of this work may help patients in the future.
“We’re looking for a better way to quantify the depth of anesthesia in the operating room and to assess consciousness in someone who has had a stroke or brain damage,” Hudetz says. “For example, we may assume that a patient is fully unconscious based on behavior, but in some cases consciousness can persist despite unresponsiveness.”
The team hopes this and future research could lead to therapeutic strategies for patients.
“We want to understand the communication breakdown that occurs in the brain during unconsciousness so we can precisely target or monitor these circuits to achieve safer anesthesia and restore these circuits to improve outcomes of coma,” Mashour says.
Estrogen Alters Memory Circuit Function in Women with Gene Variant
Fluctuations in estrogen can trigger atypical functioning in a key brain memory circuit in women with a common version of a gene, NIMH scientists have discovered. Brain scans revealed altered circuit activity linked to changes in the sex hormone in women with the gene variant while they performed a working memory task.

(Image caption: Both PET scans (left) and fMRI scans (right) showed the same atypical activation (yellow) in the brain’s memory hub, or hippocampus, in response to estrogen in women performing a working memory task – if they carried a uniquely human version of the BDNF gene. Activity in this area is typically suppressed during working memory. Picture shows PET and fMRI data superimposed over anatomical MRI image)
The findings may help to explain individual differences in menstrual cycle and reproductive-related mental disorders linked to fluctuations in the hormone. They may also shed light on mechanisms underlying sex-related differences in onset, severity, and course of mood and anxiety disorders and schizophrenia. The gene-by-hormone interaction’s effect on circuit function was found only with one of two versions of the gene that occurs in about a fourth of white women.
Drs. Karen Berman, Peter Schmidt, Shau-Ming Wei, and colleagues, of the NIMH Intramural Research Program, report on this first such demonstration in women April 18, 2017 in the journal Molecular Psychiatry.
Prior to the study, there was little evidence from research on the human brain that might account for individual differences in cognitive and behavioral effects of sex hormones. For example, why do some women develop postpartum depression and others do not – in response to the same hormone changes? Why do some women report that estrogen replacement improved their memory, whereas large studies of postmenopausal estrogen therapy show no overall improvement in memory performance?
Evidence from humans has also been lacking for the neural basis of stark sex differences in prevalence and course of mental disorders that are likely related to sex hormones. For example, why are there higher rates of mood disorders in females and higher rates of ADHD in males – or later onset of schizophrenia in females?
In seeking answers to these questions, the researchers focused on working memory, a well-researched brain function often disturbed in many of these disorders. It was known that working memory is mediated by a circuit from the brain’s executive hub, the prefrontal cortex, to its memory hub, the hippocampus. Notably, hippocampus activity is typically suppressed during working memory processing.
Following-up on a clue from experiments in mice, the NIMH team hypothesized that estrogen tweaks circuit function by interacting with a uniquely human version of the gene that codes for brain derived neurotrophic factor (BDNF), a pivotal chemical messenger operating in this circuit. To find out, the researchers experimentally manipulated estrogen levels in healthy women with one or the other version of the BDNF gene over a period of months. Researchers periodically scanned the women’s brain activity while they performed a working memory task to see any effects of the gene-hormone interaction on circuit function.
The researchers first scanned 39 women using PET (positron emission tomography) and later confirmed the results in 27 women using fMRI (functional magnetic resonance imaging). Both pegged atypical activity in the hippocampus to the interaction. Turning up the same findings using two types of neuroimaging strengthens the case for the accuracy of their observations, say the researchers. Such gene-hormone interactions affecting thinking and behavior are consistent with findings from animal studies and are suspect mechanisms conferring risk for mental illness, they add.

New Approach to Treating Alzheimer’s Disease
Alzheimer’s disease (AD) is one of the most common form of dementia. In search for new drugs for AD, the research team, led by Professor Mi Hee Lim of Natural Science at UNIST has developed a metal-based substance that works like a pair of genetic scissors to cut out amyloid-β (Aβ), the hallmark protein of AD.
The study has been featured on the cover of the January 2017 issue of the Journal of the American Chemical Society (JACS) and has been also selected as a JACS Spotlight article.
Alzheimer’s disease is the sixth leading cause of death among in older adults. The exact causes of Alzheimer’s disease are still unknown, but several factors are presumed to be causative agents. Among these, the aggregation of amyloid-β peptide (Aβ) has been implicated as a contributor to the formation of neuritic plaques, which are pathological hallmarks of Alzheimer’s disease (AD).
As therapeutics for AD, Professor Lim suggested a strategy that uses metal-based complexes for reducing the toxicity of the amyloid beta (Aβ). Althought various metal complexes have been suggested as therapeutics for AD, none of them work effectively in vivo.
The research team has found that they can hydrolyze amyloid-beta proteins using a crystal structure, called tetra-N methylated cyclam (TMC). Hydrolysis is the process that uses water molecules to split other molecules apart. The metal-mediated TMC structure uses the external water and cut off the binding of amyloid-beta protein effectively.
In this study, the following four metals (cobalt, nickel, copper and zinc) were placed at the center of the TMC structure. When the double-layered cobalt was added to the center, the hydrolysis activity was at the highest.
The research team reported that the cobalt-based metal complex (Co(II)(TMC)) had the potential to penetrate the blood brain barrier and the hydrolysis activity for nonamyloid protein was low. Moreover, the effects of this substance on the toxicity of amyloid-beta protein were also observed in living cell experiments.
“This material has a high therapeutic potential in the treatment of Alzheimer’s disease as it can penetrate the brain-vascular barrier and directly interact with the amyloid-beta protein in the brain,” says Professor Lim.
This study has also attracted attention by the editor of the Journal of the American Chemical Society. “Not only do they develop new materials, but they have been able to propose details of the working principles and experiments that support them,” according to the editor.
“As a scientist, this is such a great honor to know that our recent publication in JACS was highlighted in JACS Spotlights,” says Professor Lim. “This means that our research has not only been recognized as an important research, but also has caused a stir in academia.”

(Image caption: An fMRI scan shows regions of the brain that become active when devoutly religious study participants have a spiritual experience, including a reward center in the brain, the nucleus accumbens. Credit: Jeffrey Anderson)
This is your brain on God
Religious and spiritual experiences activate the brain reward circuits in much the same way as love, sex, gambling, drugs and music, report researchers at the University of Utah School of Medicine. The findings were published in the journal Social Neuroscience.
“We’re just beginning to understand how the brain participates in experiences that believers interpret as spiritual, divine or transcendent,” says senior author and neuroradiologist Jeff Anderson. “In the last few years, brain imaging technologies have matured in ways that are letting us approach questions that have been around for millennia.”
Specifically, the investigators set out to determine which brain networks are involved in representing spiritual feelings in one group, devout Mormons, by creating an environment that triggered participants to “feel the Spirit.” Identifying this feeling of peace and closeness with God in oneself and others is a critically important part of Mormons’ lives — they make decisions based on these feelings; treat them as confirmation of doctrinal principles; and view them as a primary means of communication with the divine.
During fMRI scans, 19 young-adult church members — including seven females and 12 males — performed four tasks in response to content meant to evoke spiritual feelings. The hour-long exam included six minutes of rest; six minutes of audiovisual control (a video detailing their church’s membership statistics); eight minutes of quotations by Mormon and world religious leaders; eight minutes of reading familiar passages from the Book of Mormon; 12 minutes of audiovisual stimuli (church-produced video of family and Biblical scenes, and other religiously evocative content); and another eight minutes of quotations.
During the initial quotations portion of the exam, participants — each a former full-time missionary — were shown a series of quotes, each followed by the question “Are you feeling the spirit?” Participants responded with answers ranging from “not feeling” to “very strongly feeling.”
Researchers collected detailed assessments of the feelings of participants, who, almost universally, reported experiencing the kinds of feelings typical of an intense worship service. They described feelings of peace and physical sensations of warmth. Many were in tears by the end of the scan. In one experiment, participants pushed a button when they felt a peak spiritual feeling while watching church-produced stimuli.
“When our study participants were instructed to think about a savior, about being with their families for eternity, about their heavenly rewards, their brains and bodies physically responded,” says lead author Michael Ferguson, who carried out the study as a bioengineering graduate student at the University of Utah.
Based on fMRI scans, the researchers found that powerful spiritual feelings were reproducibly associated with activation in the nucleus accumbens, a critical brain region for processing reward. Peak activity occurred about 1-3 seconds before participants pushed the button and was replicated in each of the four tasks. As participants were experiencing peak feelings, their hearts beat faster and their breathing deepened.
In addition to the brain’s reward circuits, the researchers found that spiritual feelings were associated with the medial prefrontal cortex, which is a complex brain region that is activated by tasks involving valuation, judgment and moral reasoning. Spiritual feelings also activated brain regions associated with focused attention.
“Religious experience is perhaps the most influential part of how people make decisions that affect all of us, for good and for ill. Understanding what happens in the brain to contribute to those decisions is really important,” says Anderson, noting that we don’t yet know if believers of other religions would respond the same way. Work by others suggests that the brain responds quite differently to meditative and contemplative practices characteristic of some eastern religions, but so far little is known about the neuroscience of western spiritual practices.
The study is the first initiative of the Religious Brain Project, launched by a group of University of Utah researchers in 2014, which aims to understand how the brain operates in people with deep spiritual and religious beliefs.
Getting Enraged By Specific Noises Has A Genuine Neurological Basis. Does the sound of whistling enrage you? How about the noise of someone eating? It now seems likely that those people who get infuriated by certain sounds might not just be being fussy, but actually have brains hardwired to produce an excessive emotional response to particular noises.

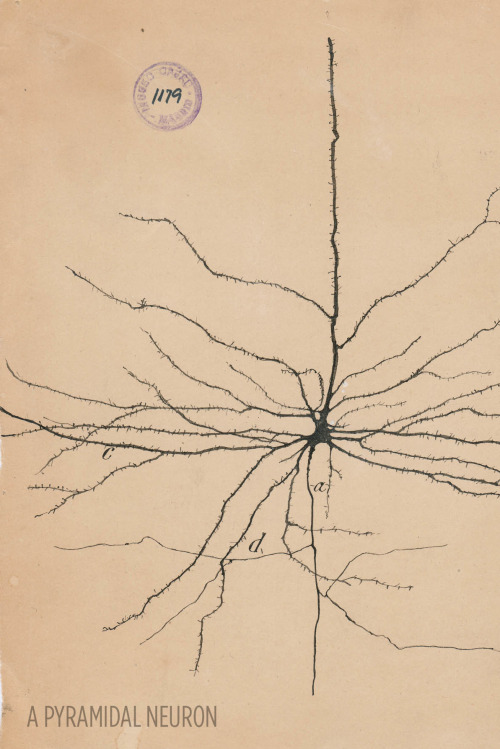



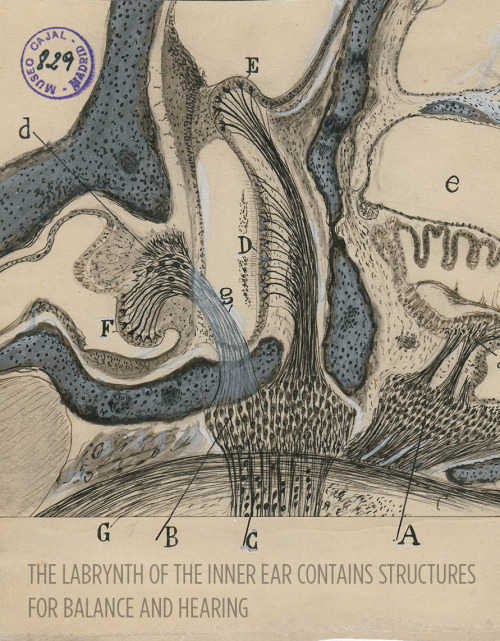


Art and science in beautiful conversation!
Here’s a 30-something Santiago Ramón y Cajal hanging out in his library:

For more, check out this article or visit the Weisman Art Museum in Minnesota before May 21.
Images courtesy of Instituto Cajal del Consjo Superior de Investigaciones Científicas, Madrid
Sounds, such as music and noise, are capable of reliably affecting individuals’ moods and emotions, possibly by regulating brain dopamine, a neurotransmitter strongly involved in emotional behavior and mood regulation.

“Sixth sense” may be more than just a feeling
With the help of two young patients with a unique neurological disorder, an initial study by scientists at the National Institutes of Health suggests that a gene called PIEZO2 controls specific aspects of human touch and proprioception, a “sixth sense” describing awareness of one’s body in space. Mutations in the gene caused the two to have movement and balance problems and the loss of some forms of touch. Despite their difficulties, they both appeared to cope with these challenges by relying heavily on vision and other senses.
“Our study highlights the critical importance of PIEZO2 and the senses it controls in our daily lives,” said Carsten G. Bönnemann, M.D., senior investigator at the NIH’s National Institute of Neurological Disorders and Stroke (NINDS) and a co-leader of the study published in the New England Journal of Medicine. “The results establish that PIEZO2 is a touch and proprioception gene in humans. Understanding its role in these senses may provide clues to a variety of neurological disorders.”
Dr. Bönnemann’s team uses cutting edge genetic techniques to help diagnose children around the world who have disorders that are difficult to characterize. The two patients in this study are unrelated, one nine and the other 19 years old. They have difficulties walking; hip, finger and foot deformities; and abnormally curved spines diagnosed as progressive scoliosis.

Working with the laboratory of Alexander T. Chesler, Ph.D., investigator at NIH’s National Center for Complementary and Integrative Health (NCCIH), the researchers discovered that the patients have mutations in the PIEZO2 gene that appear to block the normal production or activity of Piezo2 proteins in their cells. Piezo2 is what scientists call a mechanosensitive protein because it generates electrical nerve signals in response to changes in cell shape, such as when skin cells and neurons of the hand are pressed against a table. Studies in mice suggest that Piezo2 is found in the neurons that control touch and proprioception.
“As someone who studies Piezo2 in mice, working with these patients was humbling,” said Dr. Chesler. “Our results suggest they are touch-blind. The patient’s version of Piezo2 may not work, so their neurons cannot detect touch or limb movements.”
Further examinations at the NIH Clinical Center suggested the young patients lack body awareness. Blindfolding them made walking extremely difficult, causing them to stagger and stumble from side to side while assistants prevented them from falling. When the researchers compared the two patients with unaffected volunteers, they found that blindfolding the young patients made it harder for them to reliably reach for an object in front of their faces than it was for the volunteers. Without looking, the patients could not guess the direction their joints were being moved as well as the control subjects could.
The patients were also less sensitive to certain forms of touch. They could not feel vibrations from a buzzing tuning fork as well as the control subjects could. Nor could they tell the difference between one or two small ends of a caliper pressed firmly against their palms. Brain scans of one patient showed no response when the palm of her hand was brushed.
Nevertheless, the patients could feel other forms of touch. Stroking or brushing hairy skin is normally perceived as pleasant. Although they both felt the brushing of hairy skin, one claimed it felt prickly instead of the pleasant sensation reported by unaffected volunteers. Brain scans showed different activity patterns in response to brushing between unaffected volunteers and the patient who felt prickliness.
Despite these differences, the patients’ nervous systems appeared to be developing normally. They were able to feel pain, itch, and temperature normally; the nerves in their limbs conducted electricity rapidly; and their brains and cognitive abilities were similar to the control subjects of their age.
“What’s remarkable about these patients is how much their nervous systems compensate for their lack of touch and body awareness,” said Dr. Bönnemann. “It suggests the nervous system may have several alternate pathways that we can tap into when designing new therapies.”
Previous studies found that mutations in PIEZO2 may have various effects on the Piezo2 protein that may result in genetic musculoskeletal disorders, including distal arthrogryposis type 5, Gordon Syndrome, and Marden-Walker Syndrome. Drs. Bönnemann and Chesler concluded that the scoliosis and joint problems of the patients in this study suggest that Piezo2 is either directly required for the normal growth and alignment of the skeletal system or that touch and proprioception indirectly guide skeletal development.
“Our study demonstrates that bench and bedside research are connected by a two-way street,” said Dr. Chesler. “Results from basic laboratory research guided our examination of the children. Now we can take that knowledge back to the lab and use it to design future experiments investigating the role of PIEZO2 in nervous system and musculoskeletal development.”

Memory Competition
Most of the brain contains cells that no longer divide and renew. However, the dentate gyrus, nestled within the memory-forming centre of the brain (the hippocampus) is one of the few sites where new cells continue to form throughout life. As a person ages, there is an ever-increasing struggle for these new dentate gyrus neurons (coloured pink) to integrate with existing older neurons (green) because the latter already has well-established connections. This may be why learning and memorisation becomes more difficult as a person gets older. Scientists have now found that by temporarily reducing the number of dendritic spines – branches of neurons that form connections with other neurons – in the mature cells, the new cells have a better chance of functionally integrating. Indeed, in live mice, briefly eliminating dendritic spines boosted the number of integrated new neurons, which rejuvenated the hippocampus and improved the animals’ memory precision.
Written by Ruth Williams
Image courtesy of Kathleen McAvoy
Center for Regenerative Medicine, Massachusetts General Hospital, Boston, MA, USA
Copyright held by original authors
Research published in Neuron, September 2016
You can also follow BPoD on Twitter and Facebook

Network Lost
If you believe the theory of six degrees of separation, we’re all connected to each other (and possibly to Kevin Bacon) by common friends and friend-of-friends. It might feel like a small world – in fact, these patterns crops up in all sorts of places. Small world networks connect distant brain cells, and help these lymph nodes (outlined in grey) fight infections. A network of fibroblastic reticular cells (FRCs, red) spreads out inside each node, producing chemicals (green) to support immune cells while they zip around the node gathering antigens – chemical information used to target bacteria. These mouse lymph nodes are treated with different doses of a toxin that destroys FRC networks. A high dose crumples the lymph node in the bottom right. Amazingly, many of these networks repair themselves, showing just how committed immune defences are to keeping their small worlds alive.
Written by John Ankers
Image from work by Mario Novkovic, Lucas Onder and Jovana Cupovic, and colleagues
Institute of Immunobiology, Kantonsspital St. Gallen, St. Gallen, Switzerland
Image originally published under a Creative Commons Licence (BY 4.0)
Published in PLOS Biology, July 2016
You can also follow BPoD on Twitter and Facebook

(Image caption: Antidepressants move G proteins out of lipid rafts in the cell membrane. Credit: Molly Huttner)
Why do antidepressants take so long to work?
An episode of major depression can be crippling, impairing the ability to sleep, work, or eat. In severe cases, the mood disorder can lead to suicide. But the drugs available to treat depression, which can affect one in six Americans in their lifetime, can take weeks or even months to start working.
Researchers at the University of Illinois at Chicago have discovered one reason the drugs take so long to work, and their finding could help scientists develop faster-acting drugs in the future. The research was published in the Journal of Biological Chemistry.
Neuroscientist Mark Rasenick of the UIC College of Medicine and colleagues identified a previously unknown mechanism of action for selective serotonin reuptake inhibitors, or SSRIs, the most commonly prescribed type of antidepressant. Long thought to work by preventing the reabsorption of serotonin back into nerve cells, SSRIs also accumulate in patches of the cell membrane called lipid rafts, Rasenick observed, and the buildup was associated with diminished levels of an important signal molecule in the rafts.
“It’s been a puzzle for quite a long time why SSRI antidepressants can take up to two months to start reducing symptoms, especially because we know that they bind to their targets within minutes,” said Rasenick, distinguished professor of physiology and biophysics and psychiatry at UIC. “We thought that maybe these drugs have an alternate binding site that is important in the action of the drugs to reduce depressive symptoms.”
Serotonin is thought to be in short supply in people with depression. SSRIs bind to serotonin transporters – structures embedded within nerve-cell membranes that allow serotonin to pass in and out of the nerve cells as they communicate with one another. SSRIs block the transporter from ferrying serotonin that has been released into the space between neurons – the synapse – back into the neurons, keeping more of the neurotransmitter available in the synapse, amplifying its effects and reducing symptoms of depression.
Rasenick long suspected that the delayed drug response involved certain signaling molecules in nerve-cell membranes called G proteins.
Previous research by him and colleagues showed that in people with depression, G proteins tended to congregate in lipid rafts, areas of the membrane rich in cholesterol. Stranded on the rafts, the G proteins lacked access to a molecule called cyclic AMP, which they need in order to function. The dampened signaling could be why people with depression are “numb” to their environment, Rasenick reasoned.
In the lab, Rasenick bathed rat glial cells, a type of brain cell, with different SSRIs and located the G proteins within the cell membrane. He found that they accumulated in the lipid rafts over time — and as they did so, G proteins in the rafts decreased.
“The process showed a time-lag consistent with other cellular actions of antidepressants,” Rasenick said. “It’s likely that this effect on the movement of G proteins out of the lipid rafts towards regions of the cell membrane where they are better able to function is the reason these antidepressants take so long to work.”
The finding, he said, suggests how these drugs could be improved.
“Determining the exact binding site could contribute to the design of novel antidepressants that speed the migration of G proteins out of the lipid rafts, so that the antidepressant effects might start to be felt sooner.”
Rasenick already knows a little about the lipid raft binding site. When he doused rat neurons with an SSRI called escitalopram and a molecule that was its mirror image, only the right-handed form bound to the lipid raft.
“This very minor change in the molecule prevents it from binding, so that helps narrow down some of the characteristics of the binding site,” Rasenick said.

(Image caption: The brain of a fruit fly contains many different regions responsible for processing sight, smell and taste in addition to regions for controlling movement. This image shows the results of a new method which automatically identifies these brain regions. Each color represents a different brain region. The authors used this method to discover specific areas involved in processing of visual information in the fly. The technique could also be used to refine our understanding of vertebrate brains)
Identifying Brain Regions Automatically
Using the example of the fruit fly, a team of biologists led by Prof. Dr. Andrew Straw has identified patterns in the genetic activity of brain cells and taken them as a basis for drawing conclusions about the structure of the brain. The research, published in Current Biology, was conducted at the University of Freiburg and at the Research Institute of Molecular Pathology (IMP) in Vienna, Austria.
The newly developed method focuses on enhancers, DNA segments responsible for enhancing transcription of RNA at specific locations and developmental times in an organism. The research started with a database of three-dimensional images showing individual enhancer activity. The team used an automatic pattern finding algorithm to identify genetic activity patterns shared across the images. They noticed that, in some cases, these patterns seemed to correspond with specific brain regions. To demonstrate the functionality of their method, the biologists began by applying it to regions of the fruit fly brain whose anatomy is already well known – namely, those responsible for the sense of smell. The activity patterns of the enhancers traced the already familiar anatomy of these regions.
Then the biologists used the new method to study brain regions responsible for vision. These experiments led to new insights into the anatomy of these areas: In addition to eleven already known regions, the activity patterns of the enhancers revealed 14 new regions, each of which presumably serves a different function for the fruit fly’s sense of sight. The researchers now aim to conduct further studies to determine which regions are responsible for which functions.
Andrew Straw has served since January 2016 as professor of behavioral neurobiology and animal physiology at the University of Freiburg’s Faculty of Biology and is a member of the Bernstein Center Freiburg (BCF). Before their move to Freiburg, he and his research assistants Karin Panser and Dr. Laszlo Tirian worked at the Research Institute of Molecular Pathology in Vienna in collaboration with Dr. Florian Schulze, Virtual Reality and Visualization Research Center GmbH (VRVis). The goal of Straw’s research is to achieve a better understanding of the structure and function of the brain. He hopes this basic research will ultimately help in the design of therapies for patients suffering from neurological diseases affecting specific regions of the brain.
Results and visualizations: https://strawlab.org/braincode
It is imperfection - not perfection - that is the end result of the program written into that formidably complex engine that is the human brain, and of the influences exerted upon us by the environment and whoever takes care of us during the long years of physical, psychological and intellectual development.
Rita Levi-Montalcini

Image Credit: Hammersmith Hospital in London
(via neuromorphogenesis)

Manufacturing Dopamine in the Brain with Gene Therapy
Parkinson’s patients who take the drug levodopa, or L-Dopa, are inevitably disappointed. At first, during a “honeymoon” period, their symptoms (which include tremors and balance problems) are brought under control. But over time the drug becomes less effective. They may also need ultrahigh doses, and some start spending hours a day in a state of near-frozen paralysis.
A biotech company called Voyager Therapeutics now thinks it can extend the effects of L-Dopa by using a surprising approach: gene therapy. The company, based in Cambridge, Massachusetts, is testing the idea in Parkinson’s patients who’ve agreed to undergo brain surgery and an injection of new DNA.
Parkinson’s occurs when dopamine-making neurons in the brain start dying, causing movement symptoms that afflicted boxing champ Muhammad Ali and actor Michael J. Fox, whose charitable foundation has helped pay for the development of Voyager’s experimental treatment.
The cause of Parkinson’s isn’t well understood, but the reason the drug wears off is. It’s because the brain also starts losing an enzyme known as aromatic L-amino acid decarboxylase, or AADC, that is needed to convert L-Dopa into dopamine.
Voyager’s strategy, which it has begun trying on patients in a small study, is to inject viruses carrying the gene for AADC into the brain, an approach it thinks can “turn back the clock” so that L-Dopa starts working again in advanced Parkinson’s patients as it did in their honeymoon periods.
Videos of patients before and after taking L-Dopa make it obvious why they’d want the drug to work at a lower dose. In the ‘off’ state, people move in slow motion. Touching one’s nose takes an effort. In an ‘on’ state, when the drug is working, they’re shaky, but not nearly so severely disabled.
“They do well at first but then respond very erratically to L-Dopa,” says Krystof Bankiewicz, the University of California scientist who came up with the gene-therapy plan and is a cofounder of Voyager. “This trial is to restore the enzyme and allow them to be awakened, or ‘on,’ for a longer period of time.”
Voyager was formed in 2013 and later went public, raising about $86 million. The company is part of a wave of biotechs that have been able to raise money for gene therapy, a technology that is starting to pay off: after three decades of research, a few products are reaching the market.
Unlike conventional drug studies, those involving gene therapy often come with very high expectations that the treatment will work. That’s because it corrects DNA errors for which the exact biological consequences are known. Genzyme, a unit of the European drug manufacturer Sanofi, paid Voyager $65 million and promised hundreds of millions more in order to sell any treatments it develops in Europe and Asia.
“We’re working with 60 years of dopamine pharmacology,” says Steven Paul, Voyager’s CEO, and formerly an executive at the drug giant Eli Lilly. “If we can get the gene to the right tissue at the right time, it would be surprising if it didn’t work.”
But those are big ifs. In fact, the concept for the Parkinson’s gene therapy dates to 1986, when Bankiewicz first determined that too little AADC was the reason L-Dopa stops working. He thought gene therapy might be a way to fix that, but it wasn’t until 20 years later that he was able to test the idea in 10 patients, in a study run by UCSF.
In that trial, Bankiewicz says, the gene delivery wasn’t as successful as anticipated. Not enough brain cells were updated with the new genetic information, which is shuttled into them by viruses injected into the brain. Patients seemed to improve, but not by much.
Even though the treatment didn’t work as planned, that early study highlighted one edge Voyager’s approach has over others. It is possible to tag AADC with a marker chemical, so doctors can actually see it working inside patients’ brains. In fact, ongoing production of the dopamine-making enzyme is still visible in the brains of the UCSF patients several years later.

It is possible to tag AADC with a marker chemical, so doctors can actually see it working inside patients’ brains. Image Source: MIT Technology Review.
In some past studies of gene therapy, by contrast, doctors had to wait until patients died to find out whether the treatment had been delivered correctly. “This is a one-and-done treatment,” says Paul. “And anatomically, it tells us if we got it in the right place.”
A new trial under way, this one being carried out by Voyager, is designed to get much higher levels of DNA into patients’ brains in hopes of achieving better results. To do that, Bankiewicz developed a system to inject the gene-laden viral particles through pressurized tubes while a patient lies inside an MRI scanner. That way, the surgeon can see the putamen, the brain region where the DNA is meant to end up, and make sure it’s covered by the treatment.
There are other gene therapies for Parkinson’s disease planned or in testing. A trial developed at the National Institutes of Health seeks to add a growth factor and regenerate cells. A European company, Oxford BioMedica, is trying to replace dopamine.
Altogether, as of this year, there were 48 clinical trials under way of gene or cell replacement in the brain and nervous system, according to the Alliance for Regenerative Medicine, a trade group. The nervous system is the fourth most common target for this style of experimental treatment, after cancer, heart disease, and infections.
Voyager’s staff is enthusiastic about a study participant they call “patient number 6,” whom they’ve been tracking for several months—ever since he got the treatment. Before the gene therapy, he was on a high dose of L-Dopa but still spent six hours a day in an “off” state. Now he’s off only two hours a day and takes less of the drug.
That patient got the highest dose of DNA yet, covering the largest brain area. That is part of what makes Voyager think higher doses should prove effective. “I believe that previous failure of gene-therapy trials in Parkinson’s was due to suboptimal delivery,” says Bankiewicz.
Image Credit: L.A. JOHNSON
Source: MIT Technology Review (by Antonio Regalado)

At last, we’ve seen what might be the primary building blocks of memories lighting up in the brains of mice.
We have cells in our brains – and so do rodents – that keep track of our location and the distances we’ve travelled. These neurons are also known to fire in sequence when a rat is resting, as if the animal is mentally retracing its path – a process that probably helps memories form, says Rosa Cossart at the Institut de Neurobiologie de la Méditerranée in Marseille, France.
But without a way of mapping the activity of a large number of these individual neurons, the pattern that these replaying neurons form in the brain has been unclear. Researchers have suspected for decades that the cells might fire together in small groups, but nobody could really look at them, says Cossart.
To get a look, Cossart and her team added a fluorescent protein to the neurons of four mice. This protein fluoresces the most when calcium ions flood into a cell – a sign that a neuron is actively firing. The team used this fluorescence to map neuron activity much more widely than previous techniques, using implanted electrodes, have been able to do.
Observing the activity of more than 1000 neurons per mouse, the team watched what happened when mice walked on a treadmill or stood still.
As expected, when the mice were running, the neurons that trace how far the animal has travelled fired in a sequential pattern, keeping track.
These same cells also lit up while the mice were resting, but in a strange pattern. As they reflected on their memories, the neurons fired in the same sequence as they had when the animals were running, but much faster. And rather than firing in turn individually, they fired together in sequential blocks that corresponded to particular fragments of a mouse’s run.
“We’ve been able to image the individual building-blocks of memory,” Cossart says, each one reflecting a chunk of the original episode that the mouse experienced.
Continue Reading.

Shocking New Role Found for the Immune System: Controlling Social Interactions
In a startling discovery that raises fundamental questions about human behavior, researchers at the University of Virginia School of Medicine have determined that the immune system directly affects – and even controls – creatures’ social behavior, such as their desire to interact with others.
So could immune system problems contribute to an inability to have normal social interactions? The answer appears to be yes, and that finding could have significant implications for neurological diseases such as autism-spectrum disorders and schizophrenia.
“The brain and the adaptive immune system were thought to be isolated from each other, and any immune activity in the brain was perceived as sign of a pathology. And now, not only are we showing that they are closely interacting, but some of our behavior traits might have evolved because of our immune response to pathogens,” explained Jonathan Kipnis, chair of UVA’s Department of Neuroscience. “It’s crazy, but maybe we are just multicellular battlefields for two ancient forces: pathogens and the immune system. Part of our personality may actually be dictated by the immune system.”
Evolutionary Forces at Work
It was only last year that Kipnis, the director of UVA’s Center for Brain Immunology and Glia, and his team discovered that meningeal vessels directly link the brain with the lymphatic system. That overturned decades of textbook teaching that the brain was “immune privileged,” lacking a direct connection to the immune system. The discovery opened the door for entirely new ways of thinking about how the brain and the immune system interact.

(Image caption: Normal brain activity, left, and a hyper-connected brain. Credit: Anita Impagliazzo, UVA Health System)
The follow-up finding is equally illuminating, shedding light on both the workings of the brain and on evolution itself. The relationship between people and pathogens, the researchers suggest, could have directly affected the development of our social behavior, allowing us to engage in the social interactions necessary for the survival of the species while developing ways for our immune systems to protect us from the diseases that accompany those interactions. Social behavior is, of course, in the interest of pathogens, as it allows them to spread.
The UVA researchers have shown that a specific immune molecule, interferon gamma, seems to be critical for social behavior and that a variety of creatures, such as flies, zebrafish, mice and rats, activate interferon gamma responses when they are social. Normally, this molecule is produced by the immune system in response to bacteria, viruses or parasites. Blocking the molecule in mice using genetic modification made regions of the brain hyperactive, causing the mice to become less social. Restoring the molecule restored the brain connectivity and behavior to normal. In a paper outlining their findings, the researchers note the immune molecule plays a “profound role in maintaining proper social function.”
“It’s extremely critical for an organism to be social for the survival of the species. It’s important for foraging, sexual reproduction, gathering, hunting,” said Anthony J. Filiano, Hartwell postdoctoral fellow in the Kipnis lab and lead author of the study. “So the hypothesis is that when organisms come together, you have a higher propensity to spread infection. So you need to be social, but [in doing so] you have a higher chance of spreading pathogens. The idea is that interferon gamma, in evolution, has been used as a more efficient way to both boost social behavior while boosting an anti-pathogen response.”
Understanding the Implications
The researchers note that a malfunctioning immune system may be responsible for “social deficits in numerous neurological and psychiatric disorders.” But exactly what this might mean for autism and other specific conditions requires further investigation. It is unlikely that any one molecule will be responsible for disease or the key to a cure. The researchers believe that the causes are likely to be much more complex. But the discovery that the immune system – and possibly germs, by extension – can control our interactions raises many exciting avenues for scientists to explore, both in terms of battling neurological disorders and understanding human behavior.
“Immune molecules are actually defining how the brain is functioning. So, what is the overall impact of the immune system on our brain development and function?” Kipnis said. “I think the philosophical aspects of this work are very interesting, but it also has potentially very important clinical implications.”
Findings Published
Kipnis and his team worked closely with UVA’s Department of Pharmacology and with Vladimir Litvak’s research group at the University of Massachusetts Medical School. Litvak’s team developed a computational approach to investigate the complex dialogue between immune signaling and brain function in health and disease.
“Using this approach we predicted a role for interferon gamma, an important cytokine secreted by T lymphocytes, in promoting social brain functions,” Litvak said. “Our findings contribute to a deeper understanding of social dysfunction in neurological disorders, such as autism and schizophrenia, and may open new avenues for therapeutic approaches.”
The findings have been published online by the prestigious journal Nature.

(Image caption: Brandeis University professor Ricardo Godoy conducts the experiment in a village in the Bolivian rainforest. The participants were asked to rate the pleasantness of various sounds, and Godoy recorded their response. Credit: Alan Schultz)
Why we like the music we do
In Western styles of music, from classical to pop, some combinations of notes are generally considered more pleasant than others. To most of our ears, a chord of C and G, for example, sounds much more agreeable than the grating combination of C and F# (which has historically been known as the “devil in music”).
For decades, neuroscientists have pondered whether this preference is somehow hardwired into our brains. A new study from MIT and Brandeis University suggests that the answer is no.
In a study of more than 100 people belonging to a remote Amazonian tribe with little or no exposure to Western music, the researchers found that dissonant chords such as the combination of C and F# were rated just as likeable as “consonant” chords, which feature simple integer ratios between the acoustical frequencies of the two notes.
“This study suggests that preferences for consonance over dissonance depend on exposure to Western musical culture, and that the preference is not innate,” says Josh McDermott, the Frederick A. and Carole J. Middleton Assistant Professor of Neuroscience in the Department of Brain and Cognitive Sciences at MIT.
McDermott and Ricardo Godoy, a professor at Brandeis University, led the study, which appeared in Nature on July 13. Alan Schultz, an assistant professor of medical anthropology at Baylor University, and Eduardo Undurraga, a senior research associate at Brandeis’ Heller School for Social Policy and Management, are also authors of the paper.
Consonance and dissonance
For centuries, some scientists have hypothesized that the brain is wired to respond favorably to consonant chords such as the fifth (so-called because one of the notes is five notes higher than the other). Musicians in societies dating at least as far back as the ancient Greeks noticed that in the fifth and other consonant chords, the ratio of frequencies of the two notes is usually based on integers — in the case of the fifth, a ratio of 3:2. The combination of C and G is often called “the perfect fifth.”
Others believe that these preferences are culturally determined, as a result of exposure to music featuring consonant chords. This debate has been difficult to resolve, in large part because nowadays there are very few people in the world who are not familiar with Western music and its consonant chords.
“It’s pretty hard to find people who don’t have a lot of exposure to Western pop music due to its diffusion around the world,” McDermott says. “Most people hear a lot of Western music, and Western music has a lot of consonant chords in it. It’s thus been hard to rule out the possibility that we like consonance because that’s what we’re used to, but also hard to provide a definitive test.”
In 2010, Godoy, an anthropologist who has been studying an Amazonian tribe known as the Tsimane for many years, asked McDermott to collaborate on a study of how the Tsimane respond to music. Most of the Tsimane, a farming and foraging society of about 12,000 people, have very limited exposure to Western music.
“They vary a lot in how close they live to towns and urban centers,” Godoy says. “Among the folks who live very far, several days away, they don’t have too much contact with Western music.”
The Tsimane’s own music features both singing and instrumental performance, but usually by only one person at a time.
Dramatic differences
The researchers did two sets of studies, one in 2011 and one in 2015. In each study, they asked participants to rate how much they liked dissonant and consonant chords. The researchers also performed experiments to make sure that the participants could tell the difference between dissonant and consonant sounds, and found that they could.
The team performed the same tests with a group of Spanish-speaking Bolivians who live in a small town near the Tsimane, and residents of the Bolivian capital, La Paz. They also tested groups of American musicians and nonmusicians.
“What we found is the preference for consonance over dissonance varies dramatically across those five groups,” McDermott says. “In the Tsimane it’s undetectable, and in the two groups in Bolivia, there’s a statistically significant but small preference. In the American groups it’s quite a bit larger, and it’s bigger in the musicians than in the nonmusicians.”
When asked to rate nonmusical sounds such as laughter and gasps, the Tsimane showed similar responses to the other groups. They also showed the same dislike for a musical quality known as acoustic roughness.
The findings suggest that it is likely culture, and not a biological factor, that determines the common preference for consonant musical chords, says Brian Moore, a professor of psychology at Cambridge University, who was not involved in the study.
“Overall, the results of this exciting and well-designed study clearly suggest that the preference for certain musical intervals of those familiar with Western music depends on exposure to that music and not on an innate preference for certain frequency ratios,” Moore says.

A New Way to Cross the Blood–Brain Barrier - A Mental Unblock
The brain presents a unique challenge for medical treatment: it is locked away behind an impenetrable layer of tightly packed cells. Although the blood-brain barrier prevents harmful chemicals and bacteria from reaching our control center, it also blocks roughly 95 percent of medicine delivered orally or intravenously. As a result, doctors who treat patients with neurodegenerative diseases, such as Parkinson’s, often have to inject drugs directly into the brain, an invasive approach that requires drilling into the skull.
Some scientists have had minor successes getting intravenous drugs past the barrier with the help of ultrasound or in the form of nanoparticles, but those methods can target only small areas. Now neuroscientist Viviana Gradinaru and her colleagues at the California Institute of Technology show that a harmless virus can pass through the barricade and deliver treatment throughout the brain.
Gradinaru’s team turned to viruses because the infective agents are small and adept at entering cells and hijacking the DNA within. They also have protein shells that can hold beneficial deliveries, such as drugs or genetic therapies. To find a suitable virus to enter the brain, the researchers engineered a strain of an adeno-associated virus into millions of variants with slightly different shell structures. They then injected these variants into a mouse and, after a week, recovered the strains that made it into the brain. A virus named AAV-PHP.B most reliably crossed the barrier.
Next the team tested to see if AAV-PHP.B could work as a potential vector for gene therapy, a technique that treats diseases by introducing new genes into cells or by replacing or inactivating genes already there. The scientists injected the virus into the bloodstream of a mouse. In this case, the virus was carrying genes that encoded green fluorescent proteins. So if the virus made it to the brain and the new DNA was incorporated in neurons, the success rate could be tracked via a green glow on dissection. Indeed, the researchers observed that the virus infiltrated most brain cells and that the glowing effects lasted as long as one year. The results were recently published in Nature Biotechnology.
In the future, this approach could be used to treat a range of neurological diseases. “The ability to deliver genes to the brain without invasive methods will be extremely useful as a research tool. It has tremendous potential in the clinic as well,” says Anthony Zador, a neuroscientist who studies brain wiring at Cold Spring Harbor Laboratory. Gradinaru also thinks the method is a good candidate for targeting areas other than the brain, such as the peripheral nervous system. The sheer number of peripheral nerves has made pain treatment for neuropathy difficult, and a virus could infiltrate them all.
Image Credit: Thomas Fuchs
Source: Scientific American (By Monique Brouillette)

5 sleep disorders you didn’t know existed
Ever shouted at your partner while you slept, or woken up unable to move? From apnoea to exploding heads, here are some strange things that go bump in the night.
Sleep apnoea
A surprisingly common condition in which you stop breathing for 10 seconds or more as you sleep. The lack of oxygen causes your brain to wake you up, or pull you into much lighter sleep. Either way, it can have a profound effect on the quality of your sleep – and that of any bedfellow, as it’s often accompanied by loud snoring.
Sleep paralysis
A terrifying experience, where the body, which naturally becomes paralysed duringREM sleep, is still paralysed when you wake. You are fully conscious but cannot move or speak, sometimes for several minutes. Some people also feel as if they are choking or their chest is being crushed and they may have visual hallucinations. The condition can be exacerbated by sleep deprivation, some drugs, and disorders such as sleep apnoea.
Hypnagogic jerks
Those jumps or twitches you experience as you nod off, often accompanied by the sensation of falling. The cause remains a mystery. One idea is that you start dreaming before your body becomes paralysed. Another is that the twitches are a by-product of your nervous system relaxing as you drift off.
REM sleep disorder
If you’ve ever punched or shouted at your partner in the night, only to remember nothing next morning, you may have been in the grip of this condition. Here, the body isn’t fully paralysed during REM sleep, so people act out their dreams. Thistends to happen only with bad dreams.
Exploding head syndrome
This entails the sensation of a loud noise, like an exploding bomb or a gunshot, as you drift off or wake up. It affects about 1 in 10 of us and it tends to start around age 50. Nobody knows what causes it– perhaps physical changes in the middle ear, or a minor seizure in the brain’s temporal lobe. Despite its name, the condition is harmless.
Image Credit: Toby Leigh
Source: New Scientist (By Catherine de Lange)


The Red Hot Debate about Transmissible Alzheimer’s
In the 25 years that John Collinge has studied neurology, he has seen hundreds of human brains. But the ones he was looking at under the microscope in January 2015 were like nothing he had seen before.
He and his team of pathologists were examining the autopsied brains of four people who had once received injections of growth hormone derived from human cadavers. It turned out that some of the preparations were contaminated with a misfolded protein—a prion—that causes a rare and deadly condition called Creutzfeldt–Jakob disease (CJD), and all four had died in their 40s or 50s as a result. But for Collinge, the reason that these brains looked extraordinary was not the damage wrought by prion disease; it was that they were scarred in another way. “It was very clear that something was there beyond what you’d expect,” he says. The brains were spotted with the whitish plaques typical of people with Alzheimer’s disease. They looked, in other words, like young people with an old person’s disease.
For Collinge, this led to a worrying conclusion: that the plaques might have been transmitted, alongside the prions, in the injections of growth hormone—the first evidence that Alzheimer’s could be transmitted from one person to another. If true, that could have far-reaching implications: the possibility that ‘seeds’ of the amyloid-β protein involved in Alzheimer’s could be transferred during other procedures in which fluid or tissues from one person are introduced into another, such as blood transfusions, organ transplants and other common medical procedures.
Collinge felt a duty to inform the public quickly. And that’s what he did, publishing the study inNature in September, to headlines around the world. “Can you CATCH Alzheimer’s?” asked Britain’s Daily Mail, about the “potentially explosive new study”. Collinge has been careful to temper the alarm. “Our study does not mean that Alzheimer’s is actually contagious,” he stresses. Carers won’t catch it on the job, nor family members, however close. “But it raises concern that some medical procedures could be inadvertently transferring amyloid-β seeds.”
Since then, the headlines have died away, but the academic work and discussion have taken off. Could seeds of amyloid-β proteins really be transmitted and, if so, are they harmless or do they cause disease? And could seeds of other related diseases involving misfolded proteins be transmitted in a similar way? In the past decade or so, evidence has been mounting for a controversial theory that rogue proteins, known collectively as amyloids and associated with diverse neurodegenerative diseases—from Alzheimer’s to Parkinson’s and Huntington's—might share some properties of prions, including their transmissibility. Collinge’s data bolstered that theory.
Urgent though these questions are, it could take years to find answers. The paper by Collinge and his colleagues has sparked a worldwide hunt for similar amyloid pathology in autopsied brains, and a small study published in January 2016 revealed a handful of related cases. Researchers are also trying to work out what the putative amyloid seeds look like, and whether different 'strains’ of amyloids exist that are particularly damaging.
Some researchers say that it is much too early to be alarmed. They point out that the number of patients in Collinge’s study was tiny, that none had displayed symptoms of Alzheimer’s disease before their death and that another toxic protein called tau also seems to be required to cause the condition. “We have to remember that there is no conclusive evidence that seeds of amyloids can transmit actual disease or that amyloids spread in the brain in a prion-like way,” says Pierluigi Nicotera, scientific director of the German Centre for Neurodegenerative Diseases in Bonn. “There may be other biological explanations.”
Right now, there are few solid answers, but plenty of concerns. The sceptics worry that they might one day find themselves working under tight biosecurity regulations to handle proteins that they view as relatively innocuous. Others feel that the dangers may have been underestimated, and that scientists have a duty to investigate this as quickly as they can. “In my opinion, all amyloids should be considered dangerous until proven safe,” says prion and amyloid researcher Adriano Aguzzi at the University Hospital Zurich in Switzerland.
DANGEROUS FOLDS
A few decades ago, it was almost inconceivable that a protein, which has no genetic material or any other obvious way to self-replicate, could cause infectious disease. But that changed in 1982, when Stanley Prusiner, now at the University of California, San Francisco, introduced evidence for disease-causing prions, coining the term from the words 'proteinacious’ and 'infectious’. Prusiner showed that prion proteins (PrP) exist in a normal cellular form, and in a misfolded infectious form. The misfolded form causes the normal protein to also misfold, creating a cascade that overwhelms and kills cells. It cause animal brains to turn into a spongy mess in scrapie, a disease of sheep, and in bovine spongiform encephalopathy (BSE or 'mad cow disease’), as well as in human prion diseases such as CJD.
Prusiner and others also investigated how prions could spread. They showed that injecting brain extracts containing infectious prions into healthy animals seeds disease. These prions can be so aggressive that in some cases, simply eating infected brains is sufficient to transmit disease. For example, many cases of variant CJD (vCJD) are now thought to have arisen in the United Kingdom in the 1990s after people ate meat from cattle that were infected with BSE.
Since then, scientists have come to appreciate that many proteins associated with neurodegenerative diseases—including amyloid-β and tau in Alzheimer’s disease and α-synuclein in Parkinson’s disease—misfold catastrophically. Structural biologists call the entire family of misfolded proteins (including PrP) amyloids. Amyloid-β clumps into whitish plaques, tau forms ribbons called tangles and α-synuclein creates fibrous deposits called inclusions.
A decade ago, these similarities prompted neuroscientist Mathias Jucker at the University of Tübingen in Germany to test whether injecting brain extracts containing misfolded amyloid-β into mice could seed an abnormal build-up of amyloid in the animals’ brains. He found that it could, and that it also worked if he injected amyloids into the muscles. “We saw no reason not to believe that if amyloid seeds entered the human brain, they would also cause amyloid pathology in the same way,” says Jucker.
This didn’t cause alarm at the time, because it wasn’t clear how an amyloid seed from the brain of someone with Alzheimer’s could be transferred into another person’s body and find its way to their brain. To investigate that, what was needed was a group of people who had been injected with material from another person, and the opportunity to examine their brains in great detail, preferably when they were still relatively young and before they might have spontaneously developed early signs of Alzheimer’s.
The CJD brains provided just that opportunity. Between 1958 and 1985, around 30,000 people worldwide received injections of growth hormone derived from the adrenal glands of cadavers to treat growth problems. Some of the preparations were contaminated with the prion that causes CJD. Like all prion diseases, CJD has a very long incubation period, but once it gets going it rages through the brain, destroying all tissue in its wake and typically killing people from their late 40s onwards. According to 2012 statistics, 226 people around the world have died from CJD as a result of prion-contaminated growth-hormone preparations.
Collinge had not set out to find a link with Alzheimer's—it emerged as part of routine work at the National Prion Clinic in London, which he heads, and where around 70% of all people in the United Kingdom who die from prion-related causes are now autopsied. The clinic routinely looks for signs of all amyloid proteins in these brains to distinguish prion disease from other conditions. It was thanks to this routine work that the cluster of unusual cases emerged of people who had clearly died of CJD, but who also had obvious signs of amyloid pathology in their grey matter and cerebral blood vessels.
As soon as he saw these brains, Collinge knew that he could get into stormy waters. Keen to strike a balance between warning of a possible public-health risk and causing unwarranted panic, he sketched a carefully worded press release that would go out from the National Prion Centre and set up hotlines for people who had been treated with growth hormone in the past. But no panic occurred: apart from one or two overwrought headlines, the news stories were fairly measured, he says. Only around ten people called the hotlines.
For scientists, however, the paper was a red flag. “As soon as the paper came out we realized the health implications and started collecting slides and paraffin blocks from patients,” says Jiri Safar, director of the National Prion Disease Pathology Surveillance Center at Case Western Reserve University in Cleveland, Ohio. Like other pathologists in countries where people had died of CJD associated with medical procedures, he rushed to check the centre’s archives of autopsied brains to see if any of them contained the ominous amyloid deposits.
The answers are not yet in. Safar says that it has not proved easy to trace brain samples in the United States, but that he is working to do so with the National Institutes of Health and the Centers for Disease Control and Prevention (CDC) in Atlanta, Georgia. Charles Duyckaerts at the Pitié-Salpêtrière Hospital in Paris, France, has now examined brain tissues from around 24 patients and is likely to report the results later this year.
A further 228 cases of CJD were caused by transplantation of prion-contaminated dura mater—the membrane surrounding the brain and spinal cord—prepared from cadavers around the world. Dura-mater preparations were regularly used in brain surgery as repair patches until the late 1990s. For the study published in January, Herbert Budka at the National Prion Diseases Reference Center at University Hospital Zurich and his colleagues examined the brains of seven such patients from Switzerland and Austria, and found that five had amyloid deposits in grey matter and blood vessels. In Japan, dementia researcher Masahito Yamada at Kanazawa University is making his way through a large number of such autopsy specimens and says that the 16 brains he has examined so far show signs of unusually high levels of amyloid deposition in cerebral blood vessels.
Yet such case studies can only ever provide circumstantial evidence that seeds of amyloid-β were transferred during the treatments. And they cannot entirely rule out the possibility that the treatments themselves—or the patients’ original medical conditions—caused the amyloid pathology. More-conclusive evidence would come from checking whether the original growth hormone and dura-mater preparations contained infectious amyloid seeds, by injecting them into animals and seeing whether this triggers disease. Most of these preparations, however, have long since disappeared. Collinge has access to some original samples of growth hormone stored by the UK Department of Health, and he is planning to analyse them for the presence of amyloid seeds and then inject them into mice. That work will take a couple of years to complete, he says.
SEEDS OF DOUBT
There is another hitch: no one knows for sure what size and shape the amyloid seeds might be. Jucker is hunting for them in an unusual source of human brain tissue that has nothing to do with CJD. A team in Bonn has collected frozen samples from more than 700 people with epilepsy who were operated on over the past 25 years to remove tissue that was driving their seizures. “It is the best source of fresh human brain tissue available at the moment,” says Jucker, who plans to scrutinize it carefully under the microscope for anything that might resemble tiny clumps or seeds of amyloid-β. The team also has records of the patients’ cognitive skills, such as language and memory skills, before and at regular intervals after the operations. This should allow Jucker’s team to correlate the presence of any amyloid-β seeds it finds with changes in the cognitive function of individual patients over time.
Scientists have shown that tau and α-synuclein can also seed pathological features in mice. In two studies, from 2012, scientists injected fibrils of α-synuclein into the brains of mice already engineered to develop some of the characteristics of Parkinson’s disease. This triggered the early onset of some of the signs and symptoms of Parkinson’s, and eventually killed the animals. A third study showed that similar injections into normal mice caused some of the neurodegeneration typical of Parkinson’s disease and the mice became less agile. In humans, α-synuclein would not necessarily turn out to be equally aggressive—mouse models of neurodegenerative diseases do not mimic human disease very closely—but scientists are taking the possibility seriously.
If the transmissibility hypothesis proves true, the implications could be severe. Amyloids stick like glue to metal surgical instruments, and normal sterilization does not remove them, so amyloid seeds might possibly be transferred during surgery. The seeds might sit in the body for years or decades before spreading into plaques, and perhaps enabling the other pathological changes needed to induce Alzheimer’s disease. Having amyloid plaques in cerebral blood vessels could be dangerous in another way, because they increase the risk that the vessel walls might break, leading to small strokes.
But if common medical procedures really increased the risk of neurodegenerative disorders, then wouldn’t that already have been detected? Not necessarily, says epidemiologist Roy Anderson at Imperial College London. “The proper epidemiological studies have not been done yet,” he says. They require very large and carefully curated databases of people with Alzheimer’s disease, which include information about the development of symptoms and autopsy data. He and his team are now studying the handful of reliable databases that exist to tease out a signal that might associate medical procedures with Alzheimer’s progression. The number of patients currently available may turn out to be too small to draw conclusions, he says, but a more definitive answer could emerge as the databases grow.
Faced with so much uncertainty, some researchers and public-health agencies have adopted a wait-and-see approach. “We are right at the beginning of this story,” says Nicotera, “and if there is one message to come out right now it is that we need more work to see if this is a relevant mechanism.” The CDC and the European Centre for Disease Prevention and Control in Solna, Sweden, say that they are keeping a cautious eye on the issue.
If further research does confirm that common neurodegenerative diseases are transmissible, what then? One immediate priority would be rigorous sterilization procedures for medical and surgical instruments that would destroy amyloids, in the way that extremely high temperatures and harsh chemicals destroy prions. Aguzzi says that funding agencies should put out calls now to researchers to develop cheap and simple sterilization methods. “It’s not very sexy science, but it is urgently needed,” he says. He also worries about the safety of researchers working with amyloids—particularly α-synuclein. “I have nightmares that someone in my lab may catch Parkinson’s,” he says. “While the story is in flux, our first duty is to protect lab workers.”
STRAIN SEEKERS
The similarities between prions and other amyloids is throwing open other avenues of research. Prions can exist as distinct strains—proteins that have the same sequence of amino acids but misfold in different ways and have distinct biological behaviours, much as different strains of a pathogenic virus can be aggressive or weak. The outbreak of vCJD in the United Kingdom in the 1990s was traced to BSE-contaminated meat because the prion strain was the same in both.
Over the past few years, research in animals has shown that different strains of amyloid-β and α-synuclein exist. And a landmark paper in 2013 reported that strains of amyloid-β with different 3D structures were associated with different disease progression in two people with Alzheimer’s. Structural biologist Robert Tycko, who led the work at the National Institute of Diabetes and Digestive and Kidney Diseases in Bethesda, Maryland, is now looking at many more brain samples from such patients.
Knowing the structures of pathological forms of amyloid seeds should help to design small molecules that bind to them and stop them doing damage, says biophysicist Ronald Melki at the Paris-Saclay Institute of Neuroscience, who works on α-synuclein strains. His lab is designing small peptides that target the seeds and mimic regions of 'chaperone’ molecules, which usually bind to proteins and help them to fold correctly. Melki’s small peptides mimic these binding regions, sticking to the amyloid proteins to stop them from aggregating further.
In the research community, much of the agitation in response to Collinge’s paper boils down to semantics. Some scientists do not like to use the word 'prion’ in connection with the amyloids associated with common neurodegenerative diseases, or to describe any of their properties as 'prion-like'—because of its connotation of infectious, deadly disease. “The public has this perception of the word 'prion’,” says Alzheimer’s researcher Brad Hyman at Harvard Medical School in Boston, Massachusetts, and this matters, even if their ideas are wrong. “One of my patients told me that she wasn’t getting any hugs any more from her husband who had read about the case in the media—that made me sad,” he says.
Others, however, feel that it is helpful to consider prions and other amyloids as being part of a single spectrum of conditions involving proteins that misfold and misbehave. It means that researchers studying prion diseases and neurodegenerative diseases, who until recently had considered their disciplines to be separate, now find themselves tackling shared questions.
Both fields are wary of raising premature alarm, even though they wonder what the future will bring. Jucker, only half-jokingly, says he could imagine a future in which people would go into hospital every ten years or so and get the amyloid seeds cleared out of their brains with antibodies. “You’d be good then to go for another decade.”
Image 1 Credit: ©iStock.com
Image 2 Credit: Juan Gaertner/Shutterstock
Source: Scientific American (By Alison Abbott, Nature magazine)
Steve Gentleman, a neuropathologist, demonstrates the process of brain dissection and preservation for research.

New insights into the molecular basis of memory
Scientists from the German Center for Neurodegenerative Diseases (DZNE) in Göttingen and Munich have shed new light on the molecular basis of memory. Their study confirms that the formation of memories is accompanied by an altered activity of specific genes. In addition, they found an unprecedented amount of evidence that supports the hypothesis that chemical labels on the backbone of the DNA (so-called DNA methylation) may be the molecular basis of long-term memory. These findings are reported in “Nature Neuroscience”.
The brain still harbours many unknowns. Basically, it is assumed that it stores experiences by altering the connections between brain cells. This ability to adapt – which is also called “plasticity” – provides the basis for memory and learning, which is the ability to draw conclusions from memories. On a molecular scale these changes are mediated by modifications of expression of specific genes that as required strengthen or weaken the connections between the brain cells.
In the current study, a research team led by Dr. Stefan Bonn and Prof. André Fischer from Göttingen, joined forces with colleagues from the DZNE’s Munich site, to examine how the activity of such genes is regulated. The scientists stimulated long-term memory in mice, by training the animals to recognise a specific test environment. Based on tissue samples, the researchers were able to discern to what extent this learning task triggered changes in the activity of the genes in the mice’s brain cells. Their focus was directed on so-called epigenetic modifications. These modifications involve the DNA and DNA associated proteins.
Epigenetic modifications
“The cell makes use of various mechanisms in order to turn genes on or off, without altering the DNA sequence itself. It’s called ‘epigenetics’,” explains Dr. Magali Hennion, a staff member of the research group of Stefan Bonn.
In principle, gene regulation can happen through methylation, whereby the backbone of the DNA is chemically labeled at specific sites. Changes in the proteins called histones that are packaging the DNA may also occur.
Hennion: “Research on epigenetic changes that are related to memory processes is still at an early stage. We look at such features, not only for the purpose of a better understanding of how memory works. We also look for potential targets for drugs that may counteract memory decline. Ultimately, our research is about therapies against Alzheimer’s and similar brain diseases.“
A code for memory contents?
In the current study the researchers found modifications, both of the histones as well as of the methylation of the DNA. However, histone modifications had little effect on the activity of genes involved in neuroplasticity. Furthermore, Bonn and his colleagues not only discovered epigenetic modifications in nerve cells, but also in non-neuronal cells of the brain.
“The relevance of non-neuronal cells for memory, is an interesting topic that we will continue to pursue“, says André Fischer, site speaker for the DZNE in Göttingen and professor at the University Medical Center Göttingen (UMG). “Furthermore, our observations suggest that neuroplasticity is to a large extent regulated by DNA methylation. Although this is not a new hypothesis, our study provides an unprecedented amount of supporting evidence for this. Thus, methylation may indeed be an important molecular constituent of long-term memory. In such a case, methylation could be a sort of code for memory content and a potential target for therapies against Alzheimer’s disease. This is an aspect that we specifically want to focus on, in further studies.”








