Memory Competition

Memory Competition
Most of the brain contains cells that no longer divide and renew. However, the dentate gyrus, nestled within the memory-forming centre of the brain (the hippocampus) is one of the few sites where new cells continue to form throughout life. As a person ages, there is an ever-increasing struggle for these new dentate gyrus neurons (coloured pink) to integrate with existing older neurons (green) because the latter already has well-established connections. This may be why learning and memorisation becomes more difficult as a person gets older. Scientists have now found that by temporarily reducing the number of dendritic spines – branches of neurons that form connections with other neurons – in the mature cells, the new cells have a better chance of functionally integrating. Indeed, in live mice, briefly eliminating dendritic spines boosted the number of integrated new neurons, which rejuvenated the hippocampus and improved the animals’ memory precision.
Written by Ruth Williams
Image courtesy of Kathleen McAvoy
Center for Regenerative Medicine, Massachusetts General Hospital, Boston, MA, USA
Copyright held by original authors
Research published in Neuron, September 2016
You can also follow BPoD on Twitter and Facebook
More Posts from Contradictiontonature and Others

The Portuguese man o’ war delivers a powerful sting to its prey—and sometimes to people—through venom-filled structures on its tentacles. It is not a jellyfish, but rather a colony of different types of zooids (small animals). Jean Louis Coutant engraved the plate for this illustration.

It’s time for #TrilobiteTuesday! During their lengthy trek through time, trilobites existed in an almost dizzying array of sizes and shapes. Perhaps no other creature in the entire history of the earth has ever displayed the diversity of design shown by these singularly distinctive arthropods. But at their heart (and yes, trilobites apparently did possess primitive but effective cardio-respiratory systems), they were all remarkably similar. Named not, as is generally surmised, for their three main body segments – cephalon (head), thorax (body) and pygidium (tail) – but rather for the three lobes that longitudinally divided their dorsal exoskeleton. Whether they were Cambrian Olenellids – such as this Olenellus romensis from Alabama – or Devonian Phacopids, most trilobites presented a fundamentally analogous body design. Such characteristics as occipital lobes, anterior margins and facial sutures (which allowed early trilobites to shed their molting shell), were shared by the majority of trilobite species, as were such exotic-sounding features as axial rings, articulating facets and pleural spines.
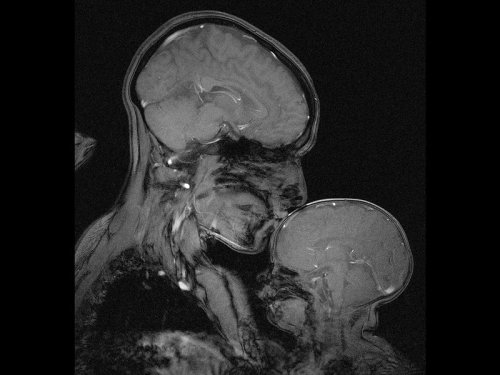
Neuroscientist captures an MRI of a mother and child
Professor Rebecca Saxe (MIT) has taken the first ever MR image of a mother and child.
“This picture is an MR image of a mother and a child that I made in my lab at MIT. You might see it as sweet and touching… an image of universal love. We can’t see clothes or hairstyles or even skin colour. From what we do see, the biology and the brains, this could be any mother and child or even father and child at any time and place in history; having an experience that any human can recognise.
Or you might see it as disturbing, a reminder that our human bodies are much too fragile as houses for ourselves. MRI’s are usually medical images and often bad news. Each white spot in that picture is a blood vessel that could clog, each tiny fold of those brains could harbour a tumour. The baby’s brain maybe looks particularly vulnerable pressed against the soft thin shell of its skull.
I see those things, universal emotions and frightening fragility but I also see one of the most amazing transformations in biology.”
Quotes have been taken from a TEDx talk given by Professor Saxe explaining the story behind the above picture.

Even after someone is declared dead, life continues in the body, suggests a surprising new study with important implications.
Gene expression — when information stored in DNA is converted into instructions for making proteinsor other molecules — actually increases in some cases after death, according to the new paper, which tracked postmortem activity and is published in the journal Open Biology.
“Not all cells are ‘dead’ when an organism dies,” senior author Peter Noble of the University of Washington and Alabama State University told Seeker. “Different cell types have different life spans, generation times and resilience to extreme stress.”
In fact, some cells seem to fight to live after the organism has died.
“It is likely that some cells remain alive and are attempting to repair themselves, specifically stem cells,” Noble said.

Neurotransmitters are chemicals that help in transmitting signals across a synapse. Different neurotransmitters are associated with different functions. Knowledge about these helps us to treat various neurological conditions by either stimulating or inhibiting these production. #neurology #neuroscience #psychiatry #medicine #medstudynotes #medschool #mbbs #unimed #brain #nervoussystem #physiology #medblog #medblr #medstudent https://www.instagram.com/p/BrM4ocsBqJe/?utm_source=ig_tumblr_share&igshid=12tojib83c32d

How the ‘police’ of the cell world deal with 'intruders’ and the 'injured’
The job of policing the microenvironment around our cells is carried out by macrophages. Macrophages are the 'guards’ that patrol most tissues of the body - poised to engulf infections or destroy and repair damaged tissue.
Over the last decade it has been established that macrophages are capable of detecting changes in the microenvironment of human tissues. They can spot pathogen invasion and tissue damage, and mediate inflammatory processes in response, to destroy microbial interlopers and remove and repair damaged tissue. But how do these sentinels of the cell world deal with infection and tissue injury?
Dr Anna Piccinini, an expert in inflammatory signalling pathways in the School of Pharmacy at The University of Nottingham, has discovered that the macrophage’s 'destroy and repair service’ is capable of discriminating between the two distinct threats even deploying a single sensor. As a result, they can orchestrate specific immune responses - passing on information in the form of inflammatory molecules and degrading tissue when they encounter an infection and making and modifying molecular components of the tissue when they detect tissue damage.
Dr Piccinini’s research is published today, Tuesday 30 August 2016, in the academic journal Science Signaling. Her findings could provide future targets for the treatment of diseases with extensive tissue damage such as arthritis or cancer where inflammation plays an increasingly recognized role.
Science Signaling
Macrophage Engulfing Bacteria, Artwork by David Mack










TOP TEN MOST DEADLY INFECTIOUS DISEASES
This list is based off of the assumption that the infected individual does not receive medical treatment.
1. Prions (mad cow disease, Creutzfeld-Jakob disease, kuru, fatal familial insomnia): 100%
2. Rabies: ~100%
3. African trypanosomiasis (’African sleeping sickness’): ~100%
4. Primary amoebic encephalitis caused by Naegleri fowlerii (’the brain-eating amoeba’): ~100%
5. Yersinia pestis, specifically the pneumonic or septicemic subtype (’the black plague’): ~100%
6. Visceral leishmaniasis: ~100%
7. Smallpox, specifically the malignant (flat) or hemorragic subtype: 95%
8. Ebola virus, specifically the Zaire strain: 83-90%
9. HIV: 80-90%
10. Anthrax, specifically the pulmonary subtype: >85%
Here’s something cool to do with your leftover candy corn – all you have to do is head to space.
Astronauts are allowed to bring special “crew preference” items when they go up in space. NASA astronaut Don Pettit chose candy corn for his five and a half month stint aboard the International Space Station. But these candy corn were more than a snack, Pettit used them for experimentation.
See how he did it:
Estrogen Alters Memory Circuit Function in Women with Gene Variant
Fluctuations in estrogen can trigger atypical functioning in a key brain memory circuit in women with a common version of a gene, NIMH scientists have discovered. Brain scans revealed altered circuit activity linked to changes in the sex hormone in women with the gene variant while they performed a working memory task.

(Image caption: Both PET scans (left) and fMRI scans (right) showed the same atypical activation (yellow) in the brain’s memory hub, or hippocampus, in response to estrogen in women performing a working memory task – if they carried a uniquely human version of the BDNF gene. Activity in this area is typically suppressed during working memory. Picture shows PET and fMRI data superimposed over anatomical MRI image)
The findings may help to explain individual differences in menstrual cycle and reproductive-related mental disorders linked to fluctuations in the hormone. They may also shed light on mechanisms underlying sex-related differences in onset, severity, and course of mood and anxiety disorders and schizophrenia. The gene-by-hormone interaction’s effect on circuit function was found only with one of two versions of the gene that occurs in about a fourth of white women.
Drs. Karen Berman, Peter Schmidt, Shau-Ming Wei, and colleagues, of the NIMH Intramural Research Program, report on this first such demonstration in women April 18, 2017 in the journal Molecular Psychiatry.
Prior to the study, there was little evidence from research on the human brain that might account for individual differences in cognitive and behavioral effects of sex hormones. For example, why do some women develop postpartum depression and others do not – in response to the same hormone changes? Why do some women report that estrogen replacement improved their memory, whereas large studies of postmenopausal estrogen therapy show no overall improvement in memory performance?
Evidence from humans has also been lacking for the neural basis of stark sex differences in prevalence and course of mental disorders that are likely related to sex hormones. For example, why are there higher rates of mood disorders in females and higher rates of ADHD in males – or later onset of schizophrenia in females?
In seeking answers to these questions, the researchers focused on working memory, a well-researched brain function often disturbed in many of these disorders. It was known that working memory is mediated by a circuit from the brain’s executive hub, the prefrontal cortex, to its memory hub, the hippocampus. Notably, hippocampus activity is typically suppressed during working memory processing.
Following-up on a clue from experiments in mice, the NIMH team hypothesized that estrogen tweaks circuit function by interacting with a uniquely human version of the gene that codes for brain derived neurotrophic factor (BDNF), a pivotal chemical messenger operating in this circuit. To find out, the researchers experimentally manipulated estrogen levels in healthy women with one or the other version of the BDNF gene over a period of months. Researchers periodically scanned the women’s brain activity while they performed a working memory task to see any effects of the gene-hormone interaction on circuit function.
The researchers first scanned 39 women using PET (positron emission tomography) and later confirmed the results in 27 women using fMRI (functional magnetic resonance imaging). Both pegged atypical activity in the hippocampus to the interaction. Turning up the same findings using two types of neuroimaging strengthens the case for the accuracy of their observations, say the researchers. Such gene-hormone interactions affecting thinking and behavior are consistent with findings from animal studies and are suspect mechanisms conferring risk for mental illness, they add.

Plantibodies and Plant-Derived Edible Vaccines
Throughout history, humans have used plants in the treatment of disease. This includes more traditional methods involving direct consumption with minimal preparation involved and the extraction of compounds for use in modern pharmaceuticals. One of the more recent methods of using plants in medicine involves the synthesis and application of plantibodies and plant produced antigens. These are recombinant antibodies and antigens respectively, which have been produced by a genetically modified plant (1, 2).
Antibodies are a diverse set of proteins which serve the purpose of aiding the body in eliminating foreign pathogens. They are secreted by effector B lymphocytes which are a type of white blood cell that circulate throughout the body. An antigen is a molecule or a component of a molecule, such as a protein or carbohydrate, which can stimulate an immune response. The human body is capable of producing around 1012 different types of antibodies, each of which can bind to a specific antigen or a small group of related motifs (3). When an antibody encounters the antigen of a foreign pathogen to which it has high affinity, it binds to it which can disable it or alert the immune system for its destruction (4).

Figure 1: Each type of antibody has the ability to bind to a specific antigen or group of antigens with high affinity.
Plants do not normally produce antibodies and thus must be genetically modified to produce plantibodies as well as foreign protein antigens. Plantibodies produced in this manner function the same way as the antibodies native to the human body (1). The main ways to do this are to stably integrate foreign DNA into a host cell and place it into a plant embryo resulting in a permanent change of the nuclear genome, or to induce transient gene expression of the specified protein (5). In both cases, the genetic material introduced to the plant codes for the protein of choice. Several of the methods used to induce permanent transgene expression include agrobacterium-mediated transformation, particle bombardment using a gene gun, or the transformation of organelles such as chloroplasts. Transient transgene expression can be done using plant viruses as viral vectors or agroinfiltration (2). Once the genetic material has been inserted, the specified protein is produced via the plant endomembrane and secretory systems, after which it can be recovered through purification of the plant tissue to be used for injection (1). The production of these proteins can also be directed to specific organs of the plant such as the seeds using targeting signals (2). Stable integration techniques are generally used for more large scale production and when the gene in question has a high level of expression, while transient techniques are used to produce a greater yield in the short term (5).

Figure 2: A gene gun being used to introduce genetic material into the leaves of a plant.
Now how can plantibodies and plant produced antigens help us as humans? The primary purpose of producing plantibodies is for the treatment of disease via immunotherapy. Immunotherapy is a method of treatment in which one’s immune response to a particular disease is enhanced. Specific plantibodies can be produced in order to target a particular disease and then be applied to patients via injection as a means of treatment (6). Doing so provides a boost to the number of antibodies against the targeted disease in the patient’s body which helps to enhance their immune system response against it. An example of this is CaroRx, the first clinically tested plantibody which has the ability to bind to Streptococcus mutans. CaroRx has been shown to be effective in the treatment of tooth decay caused by this species of bacteria (1). More recently, a plantibody known as ZMapp has shown potential in the treatment of Ebola. A study by Qiu et al showed that when administered up to 5 days after the onset of the disease, 100% of rhesus macaques that were administered the drug were shown to have recovered from its effects while all of the control group animals perished as a result of the disease (7). In addition, it has been experimentally administered to some humans who later recovered from the disease, although its role in their recovery was not fully ascertained (8).
Plant produced antigens on the other hand can be used to produce oral vaccines (9). Vaccines are typically biological mixtures containing a weakened pathogen and its antigens. Injection of this results in priming of the body’s adaptive immune system against the particular pathogen so that it can more easily recognize and respond to the threat in the future (4). By producing the antigens of targeted pathogens in plants through transgenic expression, edible vaccines can be created if the plant used is safe to eat. Tobacco, potato, and tomato plants have typically been used in past attempts to create them, showing success in both animal studies and a number of human trials. The advantages of using an oral vaccine include ease of administration and lower costs since specialised personel are not required for administration (9). In addition, oral vaccines are more effective in providing immunity against pathogens at mucosal surfaces as they can be directly applied to the gastrointestinal tract (1). The primary issue with the usage of oral vaccines is that protein antigens must avoid degradation in the stomach and intestines before they can reach the targeted sites in the body. Several solutions to this dilemma include using other biological structures such as liposomes and proteasomes as a means of delivery. This helps to prevent the proteins from being degraded by digestive enzymes and the acidic environment of the stomach before they can reach their destination (1, 9).

Figure 3: An overview of one method of producing an edible vaccine using a potato plant. A gene coding for the protein of a human pathogen is used in agrobacterium-mediated transformation to produce a transgenic potato plant. The potatoes from this plant can then serve as an edible vaccine against pathogen from which the protein originated.
There are a number of advantages to using these plant based pharmaceuticals. First of all, they can be produced on a large scale at a relatively low cost through agriculture and are convenient for long-term storage due to the resiliency and size of plant seeds (5). There is also a low risk of contamination by mammalian viruses, blood borne pathogens, and oncogenes which can remove the need for expensive removal steps (1). In addition, purification steps can be skipped if the plants used are edible and ethical problems that come with animal production can be avoided (5). The disadvantages include the potential for allergic reactions to plant antigens and contamination by pesticides and herbicides. There is also the possibility of outcrossing of transgenic pollen to weeds or related crops which would lead to non-target crops also expressing the pharmaceutical.This could lead to public concern along with the potential that other species which ingest these plants may be negatively affected (9). While plantibodies and plant produced antigens have not yet been extensively tested in clinical trials, going forward they represent a new treatment option with great promise.
References
1. Jain P, Pandey P, Jain D, Dwivedi P. Plantibody: An overview. Asian journal of Pharmacy and Life Science. 2011 Jan;1(1):87-94.
2. Stoger E, Sack M, Fischer R, Christou P. Plantibodies: applications, advantages and bottlenecks. Current Opinion in Biotechnology. 2002 Apr 1;13(2):161-166.
3. Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Molecular Biology of the Cell. 4th Edition. New York: Garland Science; 2002.
4. Parham P. The immune system. 4th Edition. New York: Garland Science; 2014.
5. Ferrante E, Simpson D. A review of the progression of transgenic plants used to produce plantibodies for human usage. J. Young Invest. 2001;4:1-0.
6. Smith MD. Antibody production in plants. Biotechnology advances. 1996 Dec 31;14(3):267-81.
7. Qiu X, Wong G, Audet J, Bello A, Fernando L, Alimonti JB, Fausther-Bovendo H, Wei H, Aviles J, Hiatt E, Johnson A. Reversion of advanced Ebola virus disease in nonhuman primates with ZMapp. Nature. 2014 Aug 29.
8. Sneed A. Know the Jargon. Scientific american. 2014 Dec 1;311(6):24-24.
9. Daniell H, Streatfield SJ, Wycoff K. Medical molecular farming: production of antibodies, biopharmaceuticals and edible vaccines in plants. Trends in plant science. 2001 May 1;6(5):219-26.
-
 re-wiredbrain-blog liked this · 8 years ago
re-wiredbrain-blog liked this · 8 years ago -
 beauvale reblogged this · 8 years ago
beauvale reblogged this · 8 years ago -
 beauvale liked this · 8 years ago
beauvale liked this · 8 years ago -
 grimcreepera-blog reblogged this · 8 years ago
grimcreepera-blog reblogged this · 8 years ago -
 grimcreepera-blog liked this · 8 years ago
grimcreepera-blog liked this · 8 years ago -
 jasmine7031 liked this · 8 years ago
jasmine7031 liked this · 8 years ago -
 mullercells reblogged this · 8 years ago
mullercells reblogged this · 8 years ago -
 dancezwithwolvez liked this · 8 years ago
dancezwithwolvez liked this · 8 years ago -
 nizdawgphd liked this · 8 years ago
nizdawgphd liked this · 8 years ago -
 kirkman62 liked this · 8 years ago
kirkman62 liked this · 8 years ago -
 cnik liked this · 8 years ago
cnik liked this · 8 years ago -
 antiknuckles liked this · 8 years ago
antiknuckles liked this · 8 years ago -
 nekoeka reblogged this · 8 years ago
nekoeka reblogged this · 8 years ago -
 redhousehead liked this · 8 years ago
redhousehead liked this · 8 years ago -
 cerebral-pleasures reblogged this · 8 years ago
cerebral-pleasures reblogged this · 8 years ago -
 wrappedinhoney liked this · 8 years ago
wrappedinhoney liked this · 8 years ago -
 zurvansworld-blog reblogged this · 8 years ago
zurvansworld-blog reblogged this · 8 years ago -
 zurvansworld-blog liked this · 8 years ago
zurvansworld-blog liked this · 8 years ago -
 eliore liked this · 8 years ago
eliore liked this · 8 years ago -
 officerrainbows-blog liked this · 8 years ago
officerrainbows-blog liked this · 8 years ago -
 lovewinslieskill reblogged this · 8 years ago
lovewinslieskill reblogged this · 8 years ago -
 lovewinslieskill liked this · 8 years ago
lovewinslieskill liked this · 8 years ago -
 altarflame liked this · 8 years ago
altarflame liked this · 8 years ago -
 stellathetumbler reblogged this · 8 years ago
stellathetumbler reblogged this · 8 years ago -
 stellathetumbler liked this · 8 years ago
stellathetumbler liked this · 8 years ago -
 sciencybrain reblogged this · 8 years ago
sciencybrain reblogged this · 8 years ago -
 leftfootism liked this · 8 years ago
leftfootism liked this · 8 years ago -
 vestigefauxpasdelinquent reblogged this · 8 years ago
vestigefauxpasdelinquent reblogged this · 8 years ago -
 vestigefauxpasdelinquent liked this · 8 years ago
vestigefauxpasdelinquent liked this · 8 years ago -
 nemogotlostinmydreams-blog liked this · 8 years ago
nemogotlostinmydreams-blog liked this · 8 years ago -
 kiynt-ergo-sum liked this · 8 years ago
kiynt-ergo-sum liked this · 8 years ago -
 fussyneuro liked this · 8 years ago
fussyneuro liked this · 8 years ago -
 trylonandperisphere reblogged this · 8 years ago
trylonandperisphere reblogged this · 8 years ago -
 trylonandperisphere liked this · 8 years ago
trylonandperisphere liked this · 8 years ago -
 theneuroscienceside-blog reblogged this · 8 years ago
theneuroscienceside-blog reblogged this · 8 years ago -
 onlyomphile liked this · 8 years ago
onlyomphile liked this · 8 years ago -
 valiantfanobservation-blog liked this · 8 years ago
valiantfanobservation-blog liked this · 8 years ago -
 panda-poes reblogged this · 8 years ago
panda-poes reblogged this · 8 years ago -
 cintyaoliv liked this · 8 years ago
cintyaoliv liked this · 8 years ago -
 tony-an-tunes liked this · 8 years ago
tony-an-tunes liked this · 8 years ago -
 cnsc17 liked this · 8 years ago
cnsc17 liked this · 8 years ago -
 dallaj-blog liked this · 8 years ago
dallaj-blog liked this · 8 years ago
A pharmacist and a little science sideblog. "Knowledge belongs to humanity, and is the torch which illuminates the world." - Louis Pasteur
215 posts