Contradictiontonature - Sapere Aude

More Posts from Contradictiontonature and Others
Using the Power of Space to Fight Cancer
From cancer research to DNA sequencing, the International Space Space is proving to be an ideal platform for medical research. But new techniques in fighting cancer are not confined to research on the space station. Increasingly, artificial intelligence is helping to “read” large datasets. And for the past 15 years, these big data techniques pioneered by our Jet Propulsion Laboratory have been revolutionizing biomedical research.
Microgravity Research on Space Station
On Earth, scientists have devised several laboratory methods to mimic normal cellular behavior, but none of them work exactly the way the body does. Beginning more than 40 years ago aboard Skylab and continuing today aboard the space station, we and our partners have conducted research in the microgravity of space. In this environment, in vitro cells arrange themselves into three-dimensional groupings, or aggregates. These aggregates more closely resemble what actually occurs in the human body. Cells in microgravity also tend to clump together more easily, and they experience reduced fluid shear stress – a type of turbulence that can affect their behavior. The development of 3D structure and enhanced cell differentiation seen in microgravity may help scientists study cell behavior and cancer development in models that behave more like tissues in the human body.

In addition, using the distinctive microgravity environment aboard the station, researchers are making further advancements in cancer therapy. The process of microencapsulation was investigated aboard the space station in an effort to improve the Earth-based technology. Microencapsulation is a technique that creates tiny, liquid-filled, biodegradable micro-balloons that can serve as delivery systems for various compounds, including specific combinations of concentrated anti-tumor drugs. For decades, scientists and clinicians have looked for the best ways to deliver these micro-balloons, or microcapsules, directly to specific treatment sites within a cancer patient, a process that has the potential to revolutionize cancer treatment.

A team of scientists at Johnson Space Center used the station as a tool to advance an Earth-based microencapsulation system, known as the Microencapsulation Electrostatic Processing System-II (MEPS-II), as a way to make more effective microcapsules. The team leveraged fluid behavior in microgravity to develop a new technique for making these microcapsules that would be more effective on Earth. In space, microgravity brought together two liquids incapable of mixing on Earth (80 percent water and 20 percent oil) in such a way that spontaneously caused liquid-filled microcapsules to form as spherical, tiny, liquid-filled bubbles surrounded by a thin, semipermeable, outer membrane. After studying these microcapsules on Earth, the team was able to develop a system to make more of the space-like microcapsules on Earth and are now performing activities leading to FDA approval for use in cancer treatment.

In addition, the ISS National Laboratory managed by the Center for the Advancement of Science in Space (CASIS) has also sponsored cancer-related investigations. An example of that is an investigation conducted by the commercial company Eli Lilly that seeks to crystallize a human membrane protein involved in several types of cancer together with a compound that could serve as a drug to treat those cancers.
“So many things change in 3-D, it’s mind-blowing – when you look at the function of the cell, how they present their proteins, how they activate genes, how they interact with other cells,” said Jeanne Becker, Ph.D., a cell biologist at Nano3D Biosciences in Houston and principal investigator for a study called Cellular Biotechnology Operations Support Systems: Evaluation of Ovarian Tumor Cell Growth and Gene Expression, also known as the CBOSS-1-Ovarian study. “The variable that you are most looking at here is gravity, and you can’t really take away gravity on Earth. You have to go where gravity is reduced.“
Crunching Big Data Using Space Knowledge

Our Jet Propulsion Laboratory often deals with measurements from a variety of sensors – say, cameras and mass spectrometers that are on our spacecraft. Both can be used to study a star, planet or similar target object. But it takes special software to recognize that readings from very different instruments relate to one another.
There’s a similar problem in cancer research, where readings from different biomedical tests or instruments require correlation with one another. For that to happen, data have to be standardized, and algorithms must be “taught” to know what they’re looking for.
Because space exploration and cancer research share a similar challenge in that they both must analyze large datasets to find meaning, JPL and the National Cancer Institute renewed their research partnership to continue developing methods in data science that originated in space exploration and are now supporting new cancer discoveries.
JPL’s methods are leading to the development of a single, searchable network of cancer data that researcher can work into techniques for the early diagnosis of cancer or cancer risk. In the time they’ve worked together, the two organizations’ efforts have led to the discovery of six new Food and Drug Administration-approved cancer biomarkers. These agency-approved biomarkers have been used in more than 1 million patient diagnostic tests worldwide.

It’s a tremendous Trilobite Tuesday!
When most of us think about trilobites, we imagine rather small creatures that inhabited the ancient seas. Indeed, most members of the more than 25,000 scientifically recognized trilobite species were less that three inches in length. Occasionally, however, paleontologists encounter a megafauna where, due to a variety of circumstances, the trilobite species were huge. One of these megafaunas can be found near the small Portuguese town of Arouca where the 450 million year-old Valongo formation produces prodigious numbers of exceptionally large Ordovician-age trilobites, such as this 41 cm Hungioides bohemicus. Other trilobite magafaunas appear sporadically around the globe, including Cambrian locations in Morocco and Devonian outcrops in Nevada.
Meet many more trilobites on the Museum website.

Autonomic nervous system
Structure and Function of the Sympathetic and Parasympathetic nervous system
The main function of the autonomic nervous system (ANS) is to assist the body in maintaining a relatively constant internal environment. For example, a sudden increase in systemic blood pressure activates the baroreceptors (those are receptors that detect physical pressure) which in turn modify the activity of the ANS so that the blood pressure is restored to its previous level [1].
The ANS is often regarded as a part of the motor system and is responsible for involuntary action and its effector organs are smooth muscle, cardiac muscle and glands. Another system, the somatic (meaning around the body) nervous system, is responsible for voluntary action in which skeletal muscle is the effector.
The ANS can further be divided into 3 parts: sympathetic, parasympathetic and enteric nervous systems [1][2], with the enteric nervous system sometimes being considered a separate entity [2]. Both parasympathetic and sympathetic nervous systems coexist and work in opposition with each other, ultimately maintaining the correct balance; the activity of one being more active depending on the situation. In a normal resting human, the parasympathetic nervous system dominates, while in a tense and stressful situation, the sympathetic nervous system switches to become dominant.

Figure 1. Structure and function of the central nervous system
This article will be focused on sympathetic and parasympathetic activity from the perspective of:
Anatomy
Biochemical
The sympathetic division provides your “fight or flight” whereas the parasympathetic division helps you to “rest and digest”
Anatomy
Higher centers that control autonomic function include the pons, medulla oblongata and hypothalamus [3].
The pons contains the micturition (urination) and respiratory center.
The medulla oblongata contains the respiratory, cardiac, vomiting, vasomotor and vasodilator centres [4].
The hypothalamus contains the highest concentration of autonomic centres [4]. It contains several centres that control autonomic activities, including heat loss, heat production and conservation, feeding and satiety, as well as fluid intake [4].

Figure 2. Locations of the autonomic control centres of the brain
All 3 structures receive input from certain sources by stimulation of nerve fibres resulting from chemical changes in blood composition like blood pH, blood glucose level, blood osmolarity and volume [4]. Notably, the hypothalamus receives input from cerebral cortex and the limbic system, a system that helps control emotional behaviour [3].
Autonomic promoter neurons are neurons that are found in the brain stem, hypothalamus or even cerebral hemispheres that project to preganglionic neurons (discussed below), where they form synapses with these neurons (5). Hence, input from the higher centres can be relayed to the motor neurons (preganglionic and then postganglionic neurons) which subsequently innervate different body tissues. Changes in the input from these centres could result in responses in those tissues.
The primary functional unit of the sympathetic and parasympathetic nervous system consists of a 2 neuron motor pathway (Figure 3), containing a preganglionic and postganglionic neurons, arranged in series.(2) The two synapse in peripheral ganglion. This clearly distinguishes autonomic motor nervous system and somatic nervous system. The somatic nervous system project from the CNS directly to innervated tissue without any intervening ganglia.(6)

Figure 3. Diagram showing the primary functional unit of the ANS
Sympathetic nervous system
Sympathetic preganglionic neurons mainly are concentrated in the lateral horn in the thoracic (T1-12) and upper lumbar (L1 &2) segments of the spinal cord (Figure 4).
The preganglionic axons leave the spinal cord in 3 ways:
Through the paravertebral ganglion
The preganglionic axon may synapse with postganglionic neurons in this ganglion or some axon may travel rostrally or caudally within the sympathetic trunk before forming synapse with a postganglionic neurons in a different paravertebral ganglion.
Through the prevertebral ganglion
Some preganglionic axons pass the paravertebral ganglion (no synapse occur) and form synapse with postganglionic neurons in prevertebral ganglion, also known as collateral ganglion.
Directly to the organs without any synapse
Some preganglionic axons pass through the sympathetic trunk (no synapse) and end directly on cells of the adrenal medulla, which are equivalent to postganglionic cell.
Parasympathetic nervous system
The parasympathetic preganglionic neurons are located in several cranial nerve nuclei in the brain stem and some are found in the S3 and S4 segments of the sacral spinal cord (Figure 4). The parasympathetic postganglionic neurons are located in cranial ganglia, including the ciliary ganglion, the pterygopalatine, submandibular ganglia, and the otic ganglion. Other ganglia are present near or in the walls of visceral organs. Similarly, the preganglionic neurons form synapse with the postganglionic neurons in the ganglia.

Figure 4. Anatomy of the ANS and how its nuerons innervate tissues
After knowing how nerves connect from the CNS to PNS and to different organs, we will now consider some of the neurotransmitters that are being released at different nerve terminals. It is the binding of these neurotransmitters to the receptors on the effectors that leads to biochemical and physiological changes. Some of the neurotransmitters in use are:
For the synapse between pre and postganglionic neurons mentioned above, the neurotransmitter that is released by the preganglionic axon terminal, is acetylcholine. The corresponding receptors are found on the postsynaptic membrane of postganglionic nerves and are nicotinic receptors.
Parasympathetic postganglionic nerve terminals also release acetylcholine.
Sympathetic postganglionic nerve terminals release mostly noradrenaline
The adrenal medulla receives direct stimulation from sympathetic preganglionic innervation, releases mainly adrenaline (80%) and some noradrenaline into the blood stream. In this case, both adrenaline and noradrenaline act as hormones as they are transported via blood circulating system to target organs instead of neuronal pathway.
Strangely, for the sympathetic postganglionic nerves that innervate the sweat glands, the nerves release acetylcholine (normally only by parasympathetic postganglionic nerve) instead.
1. H.P.Rang, J.M.Ritter, R.J.Flower GH. RANG & DALE’S Pharmacology. In: 8th ed. ELSEVIER CHURCHILL LIVINGSTONE; 2016. p. 145.
2. Bruce M. Koeppen BAS. BERNE & LEVY PHYSIOLOGY. In: 6th ed. MOSBY ELSEVIER; 2010. p. 218.
3. Cholinergic transmission [Internet]. 2015. Available from: http://www.dartmouth.edu/~rpsmith/Cholinergic_Transmission.html
4. Bruce M. Koeppen BAS. BERNE & LEVY PHYSIOLOGY. In: 6th ed. MOSBY ELSEVIER; 2016. p. 44.


COLORS OF CHEMISTRY
The bright colors of chemistry fascinate people of all ages. Hriday Bhattacharjee, a Ph.D. student in the lab of Jens Mueller at the University of Saskatchewan, assembled this showcase from compounds he prepared as well as from some synthesized by the undergraduate students he teaches. Organometallic and inorganic chemistry—the study of molecules like these that involve metal atoms—is especially colorful.
The table below the picture indicates the chemicals seen in the photo.
Submitted by Hriday Bhattacharjee
Do science. Take pictures. Win money: Enter our photo contest.

How are elements created in space, stars, and in laboratories? The latest edition of #PeriodicGraphics in C&EN takes a look! http://bit.ly/2UXWoPD http://bit.ly/2YbuBNE






My new favorite: Solvatofluorescence of Nile Red
Solvatochromism is the ability of a chemical substance to change color due to a change in solvent polarity, so it has different color in different solvents.
Also in some cases, the emission and excitation wavelength both shift depending on solvent polarity, so it fluoresces with different color depending on the solvent what it’s dissolved in. This effect is solvatofluorescence.
On the video the highly solvatochromic organic dye, Nile Red was added to different organic solvents and was diluted with another, different polarity organic solvent. As the polarity of the solution changed, the emitted color from the fluorescent dye also varied as seen on the gifs above and as seen on the video:
To help the blog, donate to Labphoto through Patreon: https://www.patreon.com/labphoto






history meme (french edition) → 7 inventions/achievements (2/7) the first vaccine for rabies by Louis Pasteur & Émile Roux
“Pasteur had, in the early 1880s, a vaccine for rabies, but he was a chemist and not a licensed physician, and potentially liable if he injured or killed a human being. In early july 1885, Joseph Meister, a nine year-old-boy, had been badly mauled and bitten by a rabid dog (…). Pasteur injected young Meister with his rabies vaccine: the boy did not develop rabies and recovered fully from his injuries. Pasteur became a hero, and the Parisian Institue which came to be named in his honour, and of which he was the first director, became the global prototype bacteriological and immunological research institute. By demonstrating beyond doubt that many diseases were transmitted by bacteria and could be prevented from becoming active by pasteurization techniques, Pasteur indeed changed the course of history.” – G. L. Geison, The Private Science of Louis Pasteur.
One of the smoothest, most beautiful color changes I’ve ever seen.
The reaction is methoxymethyl deprotection of one of my agonists with concentrated HCl in acetonitrile as my solvent. The color change doesn’t happen in THF!

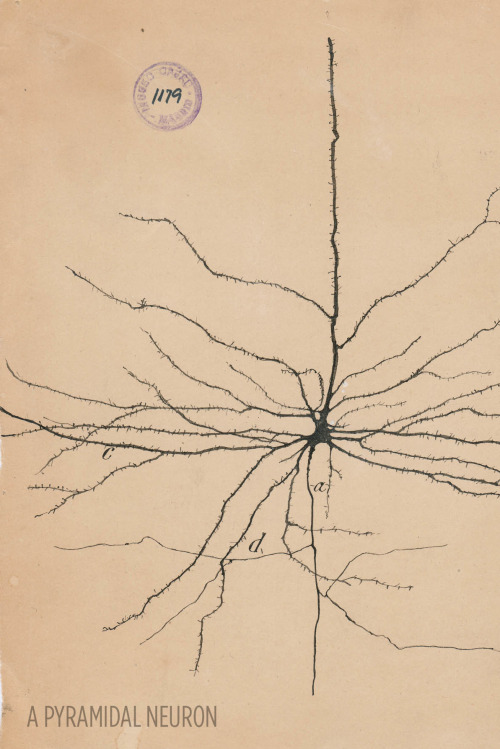



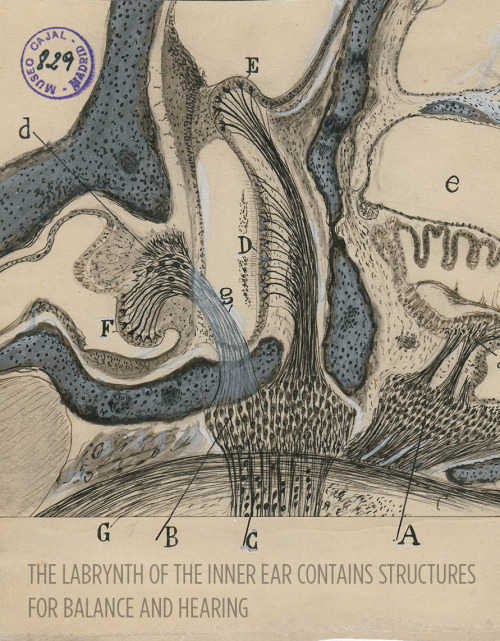


Art and science in beautiful conversation!
Here’s a 30-something Santiago Ramón y Cajal hanging out in his library:

For more, check out this article or visit the Weisman Art Museum in Minnesota before May 21.
Images courtesy of Instituto Cajal del Consjo Superior de Investigaciones Científicas, Madrid
-
 1966fe liked this · 11 months ago
1966fe liked this · 11 months ago -
 crengarrion liked this · 2 years ago
crengarrion liked this · 2 years ago -
 anti-pseudoself liked this · 3 years ago
anti-pseudoself liked this · 3 years ago -
 zhlyva liked this · 4 years ago
zhlyva liked this · 4 years ago -
 my-humble-hell liked this · 4 years ago
my-humble-hell liked this · 4 years ago -
 moonflower91 liked this · 4 years ago
moonflower91 liked this · 4 years ago -
 uofmlizard liked this · 4 years ago
uofmlizard liked this · 4 years ago -
 lifeisonegrandsong liked this · 4 years ago
lifeisonegrandsong liked this · 4 years ago -
 awokenbythebluebells reblogged this · 4 years ago
awokenbythebluebells reblogged this · 4 years ago -
 redstar-godzilla reblogged this · 4 years ago
redstar-godzilla reblogged this · 4 years ago -
 trophy-lich liked this · 4 years ago
trophy-lich liked this · 4 years ago -
 daeneryses liked this · 4 years ago
daeneryses liked this · 4 years ago -
 clueless-med reblogged this · 4 years ago
clueless-med reblogged this · 4 years ago -
 helloxith liked this · 4 years ago
helloxith liked this · 4 years ago -
 megahernandezme liked this · 4 years ago
megahernandezme liked this · 4 years ago -
 graesanatomy reblogged this · 4 years ago
graesanatomy reblogged this · 4 years ago -
 w1l50n117 reblogged this · 4 years ago
w1l50n117 reblogged this · 4 years ago -
 w1l50n117 liked this · 4 years ago
w1l50n117 liked this · 4 years ago -
 flowersandmemes1298 liked this · 4 years ago
flowersandmemes1298 liked this · 4 years ago -
 nsrahma-blog1 reblogged this · 4 years ago
nsrahma-blog1 reblogged this · 4 years ago -
 comtedemoney liked this · 4 years ago
comtedemoney liked this · 4 years ago -
 humongoushologramphilosopher liked this · 5 years ago
humongoushologramphilosopher liked this · 5 years ago -
 wassermann-2501 liked this · 5 years ago
wassermann-2501 liked this · 5 years ago -
 certainherohorsekid liked this · 5 years ago
certainherohorsekid liked this · 5 years ago -
 howtomd liked this · 5 years ago
howtomd liked this · 5 years ago -
 benrivkah liked this · 5 years ago
benrivkah liked this · 5 years ago -
 ghytajenna-blog liked this · 5 years ago
ghytajenna-blog liked this · 5 years ago -
 okupel liked this · 5 years ago
okupel liked this · 5 years ago -
 colouring-outside-the-lines liked this · 5 years ago
colouring-outside-the-lines liked this · 5 years ago -
 neue-haas-grotesk liked this · 5 years ago
neue-haas-grotesk liked this · 5 years ago -
 sentio283 liked this · 5 years ago
sentio283 liked this · 5 years ago -
 chr0matic-aberration liked this · 5 years ago
chr0matic-aberration liked this · 5 years ago -
 astraystars liked this · 5 years ago
astraystars liked this · 5 years ago -
 firlefanzerin liked this · 5 years ago
firlefanzerin liked this · 5 years ago -
 ilfumattiapascal liked this · 5 years ago
ilfumattiapascal liked this · 5 years ago -
 aristocaticirrelephantelephant liked this · 5 years ago
aristocaticirrelephantelephant liked this · 5 years ago -
 brewedj liked this · 5 years ago
brewedj liked this · 5 years ago -
 wingsandstripes liked this · 5 years ago
wingsandstripes liked this · 5 years ago -
 unicornblood-and-rosedust liked this · 5 years ago
unicornblood-and-rosedust liked this · 5 years ago -
 saturnineighth liked this · 5 years ago
saturnineighth liked this · 5 years ago -
 missira94 reblogged this · 5 years ago
missira94 reblogged this · 5 years ago -
 willstudyforbagels reblogged this · 5 years ago
willstudyforbagels reblogged this · 5 years ago
A pharmacist and a little science sideblog. "Knowledge belongs to humanity, and is the torch which illuminates the world." - Louis Pasteur
215 posts

