Redrawing The Brain’s Motor Map

Redrawing the brain’s motor map
Neuroscientists at Emory have refined a map showing which parts of the brain are activated during head rotation, resolving a decades-old puzzle. Their findings may help in the study of movement disorders affecting the head and neck, such as cervical dystonia and head tremor.
The results were published in Journal of Neuroscience.
In landmark experiments published in the 1940s and 50s, Canadian neurosurgeon Wilder Penfield and colleagues determined which parts of the motor cortex controlled the movements of which parts of the body.
Penfield stimulated the brain with electricity in patients undergoing epilepsy surgery, and used the results to draw a “motor homunculus”: a distorted representation of the human body within the brain. Penfield assigned control of the neck muscles to a region between those that control the fingers and face, a finding inconsistent with some studies that came later.
Using modern functional MRI (magnetic resonance imaging), researchers at Emory University School of Medicine have shown that the neck’s motor control region in the brain is actually between the shoulders and trunk, a location that more closely matches the arrangement of the body itself.
“We can’t be that hard on Penfield, because the number of cases where he was able to study head movement was quite limited, and studying head motion as he did, by applying an electrode directly to the brain, creates some challenges,” says lead author Buz Jinnah, MD, professor of neurology, human genetics and pediatrics at Emory University School of Medicine.
The new location for the neck muscles makes more sense, because it corresponds to a similar map Penfield established of the sense of touch (the somatosensory cortex), Jinnah says.
Participants in brain imaging studies need to keep their heads still to provide accurate data, so volunteers were asked to perform isometric muscle contraction. They attempted to rotate their heads to the left or the right, even though head movement was restricted by foam padding and restraining straps.
First author Cecilia Prudente, a graduate student in neuroscience who is now a postdoctoral associate at the University of Minnesota, developed the isometric head movement task and obtained internal funding that allowed the study to proceed.
She and Jinnah knew that isometric exercises for the wrist activated the same regions of the motor cortex as wrist movements, and used that as a reference point in their study. During brain imaging, they were able to check that particular muscles were being tensed by directly monitoring volunteers’ muscles electronically.
When volunteers contracted their neck muscles, researchers were able to detect activation in other parts of the brain too, such as the cerebellum and the basal ganglia, which are known to be involved in movement control. This comes as no surprise, Jinnah says, since these regions also control movements of the hands and other body parts.
Prudente, Jinnah and colleagues have conducted a similar study with cervical dystonia patients, with the goal of comparing the patterns of brain activation between healthy volunteers and the patients. Cervical dystonia is a painful condition in which the neck muscles contract involuntarily and the head posture is distorted.
“These results may help guide future studies in humans and animals, as well as medical or surgical interventions for cervical dystonia and other disorders involving abnormal head movements,” Prudente says.
More Posts from Science-is-magical and Others

A Falcon 9 Moon : Illuminating planet Earth’s night, full moons can have many names. This year the last full moon of northern hemisphere summer was on September 2, known to some as the Full Corn Moon. A few days earlier on August 30 this almost full moon rose just before sunset though, shining through cloudy skies over Cape Canaveral Air Force Station on Florida’s Space Coast. A well-timed snapshot caught the glare of rocket engines firing below the lunar disk, a Falcon 9 rocket’s first stage successfully returning to Cape Canaveral’s landing zone 1. About 9 minutes earlier, the same SpaceX Falcon 9 rocket had launched the SAOCOM 1B satellite toward polar orbit. The fourth launch for this reusable Falcon 9 first stage, it was the first launch to a polar orbit from Cape Canaveral since 1969. via NASA

Glutamate, an essential food for the brain
Glutamate is an amino acid with very different functions: in the pancreas, it modulates the activity of the pancreatic ß-cells responsible for insulin production, whereas in the brain it is the main excitatory neurotransmitter. In recent years, it has been suspected to play an additional role in the functioning of the brain. By discovering how the brain uses glutamate to produce energy, researchers at the University of Geneva (UNIGE) confirm this hypothesis and highlight unexpected links with the rest of the body. To read in Cell Reports.
Unlike other organs, the brain cannot draw its energy from lipids, an energy resource widely present in the body. The blood-brain barrier, which protects it from the pathogens and toxins circulating in the blood, indeed limits the passage of these lipids. Moreover, while most of the organs in the human body have the ability to store glucose by increasing their mass, the brain, prisoner of the cranial bones, cannot count on these variations in volume. Unable to store its food, it depends on sugar supplied in real-time by the rest of the body. This distribution of energy is controlled by the liver.
Pierre Maechler, professor at the Faculty of Medicine at UNIGE, and his team therefore decided to verify if glutamate was indeed an energy source for the brain. To do so, the researchers analyzed the role of the glutamate dehydrogenase enzyme in the brain. In mutant form, this enzyme, encoded by the Glud1 gene, is responsible for a congenital hyperinsulinism syndrome, a severe disease affecting at the same time the endocrine pancreas, the liver and the brain. Individuals affected by this syndrome suffer from intellectual disability and have a high risk of epilepsy. “We have suppressed the Glud1 gene in the brain of mice. In the absence of glutamate dehydrogenase, we observed that the brain was no longer able to convert glutamate into energy, even though the amino acid was present in the brain,” explains Melis Karaca, first author of this study.
Priority to the brain
Devoid of the energy supplied by cerebral glutamate, the brain sends signals to the liver to requisition a compensatory proportion of glucose, at the expense of the rest of the body. This is why the transgenic mice also showed a growth deficit and muscle atrophy. “This clearly shows how the brain works in a just-in-time manner and that each percent of energy resources is essential for its proper functioning,” highlights Professor Pierre Maechler. “If a part of this energy disappears, the brain serves itself first and the rest of the body suffers. The liver must then make more glucose by drawing upon muscle protein, resulting in loss of muscle mass. Knowing that the brain uses glutamate as an energy resource allows us to reflect on other ways to overcome a potential shortfall. ”
Scientists also suspect a correlation between the Glud1 gene and some neurodevelopmental disorders, particularly epilepsy and schizophrenia. They are currently pursuing their research by introducing in mice the same Glud1 mutation detected in epileptic patients. At the same time, another group is working with schizophrenic patients to assess the way their brain uses glutamate.






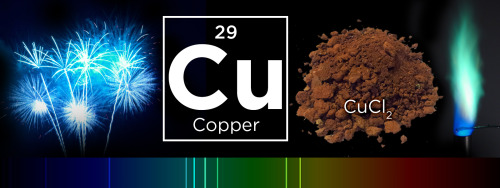

What makes fireworks colorful?
It’s all thanks to the luminescence of metals. When certain metals are heated (over a flame or in a hot explosion) their electrons jump up to a higher energy state. When those electrons fall back down, they emit specific frequencies of light - and each chemical has a unique emission spectrum.
You can see that the most prominent bands in the spectra above match the firework colors. The colors often burn brighter with the addition of an electron donor like Chlorine (Cl).
But the metals alone wouldn’t look like much. They need to be excited. Black powder (mostly nitrates like KNO3) provides oxygen for the rapid reduction of charcoal © to create a lot hot expanding gas - the BOOM. That, in turn, provides the energy for luminescence - the AWWWW.
Aluminium has a special role — it emits a bright white light … and makes sparks!
Images: Charles D. Winters, Andrew Lambert Photography / Science Source, iStockphoto, Epic Fireworks, Softyx, Mark Schellhase, Walkerma, Firetwister, Rob Lavinsky, iRocks.com, Søren Wedel Nielsen

Scientists use lasers to control mouse brain switchboard
Ever wonder why it’s hard to focus after a bad night’s sleep? Using mice and flashes of light, scientists show that just a few nerve cells in the brain may control the switch between internal thoughts and external distractions. The study, partly funded by the National Institutes of Health, may be a breakthrough in understanding how a critical part of the brain, called the thalamic reticular nucleus (TRN), influences consciousness.
“Now we may have a handle on how this tiny part of the brain exerts tremendous control over our thoughts and perceptions,” said Michael Halassa, M.D., Ph.D., assistant professor at New York University’s Langone Medical Center and a lead investigator of the study. “These results may be a gateway into understanding the circuitry that underlies neuropsychiatric disorders.”
The TRN is a thin layer of nerve cells on the surface of the thalamus, a center located deep inside the brain that relays information from the body to the cerebral cortex. The cortex is the outer, multi-folded layer of the brain that controls numerous functions, including one’s thoughts, movements, language, emotions, memories, and visual perceptions. TRN cells are thought to act as switchboard operators that control the flow of information relayed from the thalamus to the cortex.
“The future of brain research is in studying circuits that are critical for brain health and these results may take us a step further,” said James Gnadt, Ph.D., program director at NIH’s National Institute Neurological Disorders and Stroke (NINDS), which helped fund the study. “Understanding brain circuits at the level of detail attained in this study is a goal of the President’s Brain Research through Advancing Innovative Neurotechnologies (BRAIN) Initiative.”
To study the circuits, the researchers identified TRN cells that send inhibitory signals to parts of the thalamus known to relay visual information to the cortex. Using a technique known as multi-electrode recordings, they showed that sleep and concentration affected these cells in opposite ways.
They fired often when the mice were asleep, especially during short bursts of simultaneous brain cell activity called sleep spindles. These activity bursts briefly widen electrical brain wave traces making them look like spindles, the straight spikes with rounded bottoms used to make yarn. In contrast, the cells fired infrequently when the mice were tasked with using visual cues to find food. The results suggested that these cells blocked visual information from reaching the cortex during sleep and allowed its transmission when the mice were awake and attentive.
For Dr. Halassa, a practicing psychiatrist who treats schizophrenia, these surprising results may provide fundamental insights into how the brain controls information transmission, a process that is disrupted in patients with neuropsychiatric disorders. Previous studies suggested that people who experienced more spindles while sleeping were less susceptible to being disturbed by outside noises. Moreover, people with schizophrenia and autism spectrum disorder may experience fewer spindles.
“Spindles may be peepholes into the mysteries of these disorders,” said Dr. Halassa.
To test this idea, the researchers used optogenetics, a technique that introduces light-sensitive molecules into nerve cells. This allowed them to precisely control the firing patterns of visual TRN cells with flashes of laser light. The experiments were performed in well-rested as well as sleep-deprived mice. Similar to what is seen in humans, sleep deprivation can disrupt the ability of mice to focus and block out external distractions.
Well-rested mice needed just a second or two to find the food whereas sleep-deprived mice took longer, suggesting that lack of sleep had detrimental effects on their ability to focus. When the researchers used flashes of laser light to inhibit the firing of optogenetically engineered visual TRN cells in sleep-deprived mice, the mice found the food faster. In contrast, if they used optogenetics to induce sleep-like firing patterns in well-rested mice, then the mice took longer to find food.
“It’s as if with a flick of a switch we could alter the mental states of the mice and either mimic or cure their drowsiness,” said Dr. Halassa.
In a parallel set of experiments the researchers found neighbors of the visual TRN cells had very different characteristics. These neighboring cells control the flow of information to the cortex from limbic brain regions, which are involved with memory formation, emotions and arousal. The cells fired very little during sleep and instead were active when the mice were awake. Dr. Halassa thinks that their firing pattern may be important for the strengthening of new memories that often occurs during sleep. Combined, the results suggest that the TRN is divided into sub-networks that oversee discrete mental states. The researchers think understanding the sub-networks is an initial step in thoroughly exploring the role of the TRN in brain disorders.
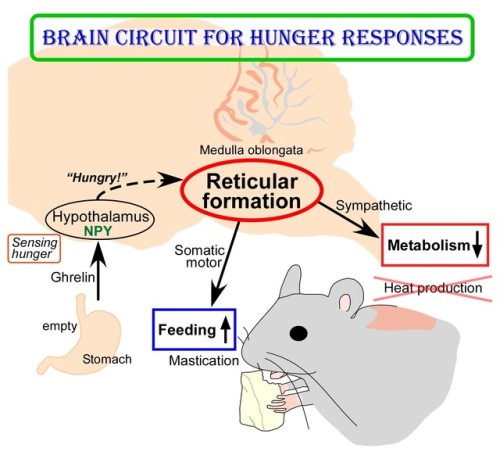
(Image caption: The empty stomach releases the hormone called ghrelin. By receiving ghrelin, the hypothalamus in the brain senses hunger and produces “hunger signaling” through the action of neuropeptide Y (NPY). The hunger signaling activates neurons in the reticular formation of the medulla oblongata, which then inhibit sympathetic output to reduce metabolic heat production and simultaneously provide masticatory motor rhythm to facilitate feeding. Credit: © 2017 Yoshiko Nakamura)
New Insights into Brain Circuit for Hunger Responses during Starvation
The human body responds to starving conditions, such as famine, to promote the chance of survival. It reduces energy expenditure by stopping heat production and promotes feeding behavior. These “hunger responses” are activated by the feeling of hunger in the stomach and are controlled by neuropeptide Y (NPY) signals released by neurons in the hypothalamus. However, how NPY signaling in the hypothalamus elicits the hunger responses has remained unknown.
Sympathetic motor neurons in the medulla oblongata are responsible for heat production by brown adipose tissue (BAT). Researchers centered at Nagoya University have now tested whether the heat-producing neurons respond to the same hypothalamic NPY signals that control hunger responses. They injected NPY into the hypothalamus of rats and tested the effect on heat production. Under normal conditions, blocking inhibitory GABAergic receptors or stimulating excitatory glutamatergic receptors in the sympathetic motor neurons induced heat production in BAT. After NPY injection, stimulating glutamatergic receptors did not produce heat, but inhibiting GABAergic receptors did. The study was reported in Cell Metabolism.
“This indicated that hypothalamic NPY signals prevent BAT thermogenesis by using inhibitory GABAergic inputs to sympathetic motor neurons,” study lead author Yoshiko Nakamura says.
Retrograde and anterograde tracing with fluorescent dyes revealed which brain region provided the inhibitory GABAergic inputs to heat-producing motor neurons.
“Tracing experiments showed that sympathetic motor neurons are directly innervated by GABAergic inputs from reticular nuclei in the medulla oblongata,” corresponding author Kazuhiro Nakamura explains, “selective activation of these GABAergic reticular neurons inhibits BAT thermogenesis.”
The researchers’ further findings showed that GABAergic inputs from medullary reticular neurons are involved in hypothalamic NPY-mediated inhibition of heat production in BAT. This hunger response circuit probably explains why anorexic individuals suffer from hypothermia.
Interestingly, stimulation of these medullary reticular neurons prompted rats to begin chewing and feeding. This effect was similar to injecting NPY into the hypothalamus, suggesting that hypothalamic NPY signaling activates reticular neurons in the medulla oblongata to promote feeding and mastication during the hunger response.
Abnormal activation of these neurons under non-starved conditions may contribute to obesity. Understanding these mechanisms could lead to development of more effective treatments for obesity.

i’m proud of them
-
 allthistime02 liked this · 3 years ago
allthistime02 liked this · 3 years ago -
 ashes086-blog1 liked this · 6 years ago
ashes086-blog1 liked this · 6 years ago -
 monseexp liked this · 7 years ago
monseexp liked this · 7 years ago -
 science-is-magical reblogged this · 8 years ago
science-is-magical reblogged this · 8 years ago -
 caterpillair-blog reblogged this · 9 years ago
caterpillair-blog reblogged this · 9 years ago -
 lecorpslanatomie-blog reblogged this · 9 years ago
lecorpslanatomie-blog reblogged this · 9 years ago -
 ossified-hypothesis liked this · 9 years ago
ossified-hypothesis liked this · 9 years ago -
 l-ange-en-rouge-archived liked this · 9 years ago
l-ange-en-rouge-archived liked this · 9 years ago -
 sophielostandfound reblogged this · 9 years ago
sophielostandfound reblogged this · 9 years ago -
 intothewoodies reblogged this · 9 years ago
intothewoodies reblogged this · 9 years ago -
 neuropath-ic reblogged this · 9 years ago
neuropath-ic reblogged this · 9 years ago -
 hermindisabubble reblogged this · 9 years ago
hermindisabubble reblogged this · 9 years ago -
 alchemist-architect liked this · 9 years ago
alchemist-architect liked this · 9 years ago -
 sexlovepleasures reblogged this · 9 years ago
sexlovepleasures reblogged this · 9 years ago -
 kommissarien-archive-blog liked this · 9 years ago
kommissarien-archive-blog liked this · 9 years ago -
 wombat-shaped liked this · 9 years ago
wombat-shaped liked this · 9 years ago -
 dramaticlemur liked this · 9 years ago
dramaticlemur liked this · 9 years ago -
 mestapleton reblogged this · 9 years ago
mestapleton reblogged this · 9 years ago -
 meanheans liked this · 9 years ago
meanheans liked this · 9 years ago -
 pocketshrikes reblogged this · 9 years ago
pocketshrikes reblogged this · 9 years ago -
 jrh8 reblogged this · 9 years ago
jrh8 reblogged this · 9 years ago -
 jrh8 liked this · 9 years ago
jrh8 liked this · 9 years ago -
 chiulaikuan liked this · 9 years ago
chiulaikuan liked this · 9 years ago -
 ah-thenah reblogged this · 9 years ago
ah-thenah reblogged this · 9 years ago -
 mmmmm0501mmmmm liked this · 9 years ago
mmmmm0501mmmmm liked this · 9 years ago -
 sauerkirshe liked this · 9 years ago
sauerkirshe liked this · 9 years ago -
 enlightenedbyscience reblogged this · 9 years ago
enlightenedbyscience reblogged this · 9 years ago -
 ghadatheadult reblogged this · 9 years ago
ghadatheadult reblogged this · 9 years ago -
 extremelypaige-blog liked this · 9 years ago
extremelypaige-blog liked this · 9 years ago -
 t1tzorgtf0 reblogged this · 9 years ago
t1tzorgtf0 reblogged this · 9 years ago -
 simplysupergirl liked this · 9 years ago
simplysupergirl liked this · 9 years ago -
 enginigger liked this · 9 years ago
enginigger liked this · 9 years ago -
 blerbzilla reblogged this · 9 years ago
blerbzilla reblogged this · 9 years ago -
 zetasinvasion liked this · 9 years ago
zetasinvasion liked this · 9 years ago -
 erdoc10 liked this · 9 years ago
erdoc10 liked this · 9 years ago -
 kevinamtab reblogged this · 9 years ago
kevinamtab reblogged this · 9 years ago -
 thesesquipedalianist liked this · 9 years ago
thesesquipedalianist liked this · 9 years ago -
 hellolydiaphillipsblog liked this · 9 years ago
hellolydiaphillipsblog liked this · 9 years ago -
 kevonkings liked this · 9 years ago
kevonkings liked this · 9 years ago -
 nasebu liked this · 9 years ago
nasebu liked this · 9 years ago -
 themightysquiggle reblogged this · 9 years ago
themightysquiggle reblogged this · 9 years ago






