(Image Caption: A New Technique Called Magnified Analysis Of Proteome (MAP), Developed At MIT, Allows

(Image caption: A new technique called magnified analysis of proteome (MAP), developed at MIT, allows researchers to peer at molecules within cells or take a wider view of the long-range connections between neurons. Credit: Courtesy of the researchers)
Imaging the brain at multiple size scales
MIT researchers have developed a new technique for imaging brain tissue at multiple scales, allowing them to peer at molecules within cells or take a wider view of the long-range connections between neurons.
This technique, known as magnified analysis of proteome (MAP), should help scientists in their ongoing efforts to chart the connectivity and functions of neurons in the human brain, says Kwanghun Chung, the Samuel A. Goldblith Assistant Professor in the Departments of Chemical Engineering and Brain and Cognitive Sciences, and a member of MIT’s Institute for Medical Engineering and Science (IMES) and Picower Institute for Learning and Memory.
“We use a chemical process to make the whole brain size-adjustable, while preserving pretty much everything. We preserve the proteome (the collection of proteins found in a biological sample), we preserve nanoscopic details, and we also preserve brain-wide connectivity,” says Chung, the senior author of a paper describing the method in the July 25 issue of Nature Biotechnology.
The researchers also showed that the technique is applicable to other organs such as the heart, lungs, liver, and kidneys.
The paper’s lead authors are postdoc Taeyun Ku, graduate student Justin Swaney, and visiting scholar Jeong-Yoon Park.
Multiscale imaging
The new MAP technique builds on a tissue transformation method known as CLARITY, which Chung developed as a postdoc at Stanford University. CLARITY preserves cells and molecules in brain tissue and makes them transparent so the molecules inside the cell can be imaged in 3-D. In the new study, Chung sought a way to image the brain at multiple scales, within the same tissue sample.
“There is no effective technology that allows you to obtain this multilevel detail, from brain region connectivity all the way down to subcellular details, plus molecular information,” he says.
To achieve that, the researchers developed a method to reversibly expand tissue samples in a way that preserves nearly all of the proteins within the cells. Those proteins can then be labeled with fluorescent molecules and imaged.
The technique relies on flooding the brain tissue with acrylamide polymers, which can form a dense gel. In this case, the gel is 10 times denser than the one used for the CLARITY technique, which gives the sample much more stability. This stability allows the researchers to denature and dissociate the proteins inside the cells without destroying the structural integrity of the tissue sample.
Before denaturing the proteins, the researchers attach them to the gel using formaldehyde, as Chung did in the CLARITY method. Once the proteins are attached and denatured, the gel expands the tissue sample to four or five times its original size.
“It is reversible and you can do it many times,” Chung says. “You can then use off-the-shelf molecular markers like antibodies to label and visualize the distribution of all these preserved biomolecules.”
There are hundreds of thousands of commercially available antibodies that can be used to fluorescently tag specific proteins. In this study, the researchers imaged neuronal structures such as axons and synapses by labeling proteins found in those structures, and they also labeled proteins that allow them to distinguish neurons from glial cells.
“We can use these antibodies to visualize any target structures or molecules,” Chung says. “We can visualize different neuron types and their projections to see their connectivity. We can also visualize signaling molecules or functionally important proteins.”
High resolution
Once the tissue is expanded, the researchers can use any of several common microscopes to obtain images with a resolution as high as 60 nanometers — much better than the usual 200 to 250-nanometer limit of light microscopes, which are constrained by the wavelength of visible light. The researchers also demonstrated that this approach works with relatively large tissue samples, up to 2 millimeters thick.
“This is, as far as I know, the first demonstration of super-resolution proteomic imaging of millimeter-scale samples,” Chung says.
“This is an exciting advance for brain mapping, a technique that reveals the molecular and connectional architecture of the brain with unprecedented detail,” says Sebastian Seung, a professor of computer science at the Princeton Neuroscience Institute, who was not involved in the research.
Currently, efforts to map the connections of the human brain rely on electron microscopy, but Chung and colleagues demonstrated that the higher-resolution MAP imaging technique can trace those connections more accurately.
Chung’s lab is now working on speeding up the imaging and the image processing, which is challenging because there is so much data generated from imaging the expanded tissue samples.
“It’s already easier than other techniques because the process is really simple and you can use off-the-shelf molecular markers, but we are trying to make it even simpler,” Chung says.
More Posts from Contradictiontonature and Others

A New Way to Cross the Blood–Brain Barrier - A Mental Unblock
The brain presents a unique challenge for medical treatment: it is locked away behind an impenetrable layer of tightly packed cells. Although the blood-brain barrier prevents harmful chemicals and bacteria from reaching our control center, it also blocks roughly 95 percent of medicine delivered orally or intravenously. As a result, doctors who treat patients with neurodegenerative diseases, such as Parkinson’s, often have to inject drugs directly into the brain, an invasive approach that requires drilling into the skull.
Some scientists have had minor successes getting intravenous drugs past the barrier with the help of ultrasound or in the form of nanoparticles, but those methods can target only small areas. Now neuroscientist Viviana Gradinaru and her colleagues at the California Institute of Technology show that a harmless virus can pass through the barricade and deliver treatment throughout the brain.
Gradinaru’s team turned to viruses because the infective agents are small and adept at entering cells and hijacking the DNA within. They also have protein shells that can hold beneficial deliveries, such as drugs or genetic therapies. To find a suitable virus to enter the brain, the researchers engineered a strain of an adeno-associated virus into millions of variants with slightly different shell structures. They then injected these variants into a mouse and, after a week, recovered the strains that made it into the brain. A virus named AAV-PHP.B most reliably crossed the barrier.
Next the team tested to see if AAV-PHP.B could work as a potential vector for gene therapy, a technique that treats diseases by introducing new genes into cells or by replacing or inactivating genes already there. The scientists injected the virus into the bloodstream of a mouse. In this case, the virus was carrying genes that encoded green fluorescent proteins. So if the virus made it to the brain and the new DNA was incorporated in neurons, the success rate could be tracked via a green glow on dissection. Indeed, the researchers observed that the virus infiltrated most brain cells and that the glowing effects lasted as long as one year. The results were recently published in Nature Biotechnology.
In the future, this approach could be used to treat a range of neurological diseases. “The ability to deliver genes to the brain without invasive methods will be extremely useful as a research tool. It has tremendous potential in the clinic as well,” says Anthony Zador, a neuroscientist who studies brain wiring at Cold Spring Harbor Laboratory. Gradinaru also thinks the method is a good candidate for targeting areas other than the brain, such as the peripheral nervous system. The sheer number of peripheral nerves has made pain treatment for neuropathy difficult, and a virus could infiltrate them all.
Image Credit: Thomas Fuchs
Source: Scientific American (By Monique Brouillette)
Meet the obscure microbe that influences climate, ocean ecosystems, and perhaps even evolution
Penny Chisholm has had a 35-year love affair—with a microbe. For her, it’s been the perfect partner—elusive during courting, a source of intellectual fulfillment, and still full of mystery decades after their introduction during an ocean cruise.
To look at, the object of her passion is just a green mote, floating in vast numbers in the world’s oceans. But Chisholm has found hidden complexity within Prochlorococcus, a cyanobacterium that is the smallest, most abundant photosynthesizing cell in the ocean—responsible for 5% of global photosynthesis, by some estimates. Its many different versions, or ecotypes, thrive from the sunlit sea surface to a depth of 200 meters, where light is minimal. Collectively the “species” boasts an estimated 80,000 genes—four times what humans have, and plenty to deal with whatever the world’s oceans throw at it. “It’s a beautiful little life machine and like a superorganism,” Chisholm says. “It’s got a story to tell us.”
Thumpety thump thump thumpety thump thump look at Kinesin go
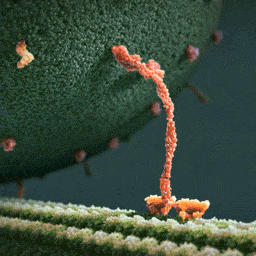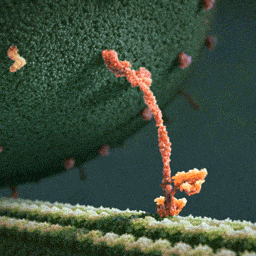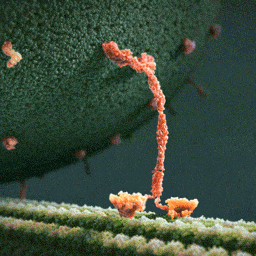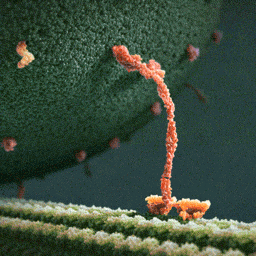
Myosin, kinesin, and dynein are important proteins governing internal transport. Myosin attached to organelles associates with actin microfilaments to enable the continuous flow of cytoplasm called cytoplasmic streaming.
Kinesins and dynein enable the movement of organelles along microtubules. They attach and move along microtubules. Most kinesins transport organelles from the center towards the periphery of the cell, anterograde transport. Dynein, and a few types of kinesins transport towards the cell center, retrograde transport.
(via thelifeofapremed)

Clear as Day
The more light your eyes can take in, the better the picture you see, and the lens at the front of your eye is transparent to help this. Most body cells contain lots of membranes – they have important roles like manufacturing cellular components, but they scatter light and aren’t transparent. Cells in the lens become transparent by losing all but their most vital internal membranes as they develop and move towards the middle of the lens: the central cells (shown here in a chick’s eye) are flatter, with rounder nuclei (blue). It wasn’t known how the membranes were lost until recently, when scientists discovered a structure called the excisosome. This forms inside cells and breaks down the membranes, possibly by stripping them apart into the proteins and lipids they’re made of. Current research implies that excisosomes form in the lenses of all animals, helping us understand how our eyes develop.
Written by Esther Redhouse White
Image from work by M.Joseph Costello and colleagues
Department of Cell Biology and Physiology, University of North Carolina, Chapel Hill, NC, USA
Image originally published under a Creative Commons Licence (BY 4.0)
Published in PLOS One, August 2016
You can also follow BPoD on Twitter and Facebook

One of the largest icebergs ever recorded, packing about a trillion tons of ice or enough to fill up two Lake Eries, has just split off from Antarctica, in a much anticipated, though not celebrated, calving event.
A section of the Larsen C ice shelf with an area of 2,240 square miles (5,800 square kilometers) finally broke away some time between July 10 and today (July 12), scientists with the U.K.-based MIDAS Project, an Antarctic research group, reported today.
Continue Reading.
DNA from the deep? Antikythera shipwreck yields ancient human bones
Death, when it came, was sudden and cruel. The individual, either a crew member or passenger, was trapped on board when the huge ship foundered. Dashed on the rocks, the vessel slid beneath the waves, tumbled down an undersea cliff, and swiftly became buried in sediment on the seabed.
Now, more than 2,000 years later, archaeologists have recovered the bones of the individual they now call Pamphilos. Thought to be a man in his late teens to early 20s, he was on the ship sailing from Asia Minor to Rome when disaster struck off the tiny Greek island of Antikythera between Crete and the Peloponnese.

WHAT??? Time to update those textbooks.
Did life begin on land rather than in the sea?
Stromatolites are round, multilayered mineral structures that range from the size of golf balls to weather balloons and represent the oldest evidence that there were living organisms on Earth 3.5 billion years ago.
Scientists who believed life began in the ocean thought these mineral formations had formed in shallow, salty seawater, just like living stromatolites in the World Heritage-listed area of Shark Bay, which is a two-day drive from the Pilbara.
But what Djokic discovered amid the strangling heat and blood-red rocks of the region was evidence that the stromatolites had not formed in salt water but instead in conditions more like the hot springs of Yellowstone.
The discovery pushed back the time for the emergence of microbial life on land by 580 million years and also bolstered a paradigm-shifting hypothesis laid out by UC Santa Cruz astrobiologists David Deamer and Bruce Damer: that life began, not in the sea, but on land.
Stromatolites.Credit: © Ints / Fotolia
Molecule of the Day: VX


VX (C11H26NO2PS) is a colourless, odourless, oily liquid under room temperatures. It is a member of the V-series of nerve agents, and is an extremely potent poison - only 0.01 grams of it is needed to kill a person by skin contact. VX was recently implicated in the assassination of Kim Jong-nam, the half-brother of the North Korean leader Kim Jong-un, in Malaysia.
VX is a potent inhibitor of acetylcholinesterase, which breaks down the neurotransmitter acetylcholine into acetic acid and choline. The normal function of the enzyme is to regulate the concentration of acetylcholine within the synaptic cleft, so as to control the frequency of binding of acetylcholine to cholinergic receptors on the postsynaptic cell membrane and hence the transmission of impulses across the synapse.

Consequently, the inhibition of acetylcholinesterase results in a rapid increase in the synaptic concentration of acetylcholine, as the presynaptic knob continues to synthesise it and secrete it into the synaptic cleft. As a result, the cholinergic receptors on the postsynaptic cell membrane are continually stimulated, and a rapid series of action potentials are triggered. This results in muscle spasms and eventual paralysis, leading to death by asphyxiation due to paralysis of the diaphragm.

VX exposure is usually treated using an injection of atropine and pralidoxime. Atropine inhibits certain cholinergic receptors, reducing the binding of acetylcholine to receptors and thus the triggering of action potentials. On the other hand, one end of pralidoxime binds to acetylcholinesterase and the other binds to the phosphate group of VX, which causes the VX molecule to detach from the enzyme together with the pralidoxime molecule (see below). This restores the ability of acetylcholinesterase to hydrolyse acetylcholine, hence reducing its synaptic levels.

VX is synthesised from phosphorus trichloride over multiple steps; first, it is methylated, reacted with ethanol, then transesterified with N,N-diisopropylaminoethanol to produce QL. This is then oxidised with sulfur, and isomerised via heating to produce VX.

-
 czkavka reblogged this · 7 years ago
czkavka reblogged this · 7 years ago -
 thoths-foundry-blog reblogged this · 8 years ago
thoths-foundry-blog reblogged this · 8 years ago -
 susucharlie liked this · 8 years ago
susucharlie liked this · 8 years ago -
 mullercells reblogged this · 8 years ago
mullercells reblogged this · 8 years ago -
 mullercells liked this · 8 years ago
mullercells liked this · 8 years ago -
 ddinthelab liked this · 8 years ago
ddinthelab liked this · 8 years ago -
 xxvi0lent-vahlkampfiidaexx liked this · 8 years ago
xxvi0lent-vahlkampfiidaexx liked this · 8 years ago -
 dandelionzola liked this · 8 years ago
dandelionzola liked this · 8 years ago -
 superjaybird liked this · 8 years ago
superjaybird liked this · 8 years ago -
 ciel62 liked this · 8 years ago
ciel62 liked this · 8 years ago -
 transwhore-blog1 liked this · 8 years ago
transwhore-blog1 liked this · 8 years ago -
 gonnabeadoc-blog reblogged this · 8 years ago
gonnabeadoc-blog reblogged this · 8 years ago -
 doomroar liked this · 8 years ago
doomroar liked this · 8 years ago -
 contradictiontonature reblogged this · 8 years ago
contradictiontonature reblogged this · 8 years ago -
 letters-from-the-sky1 reblogged this · 8 years ago
letters-from-the-sky1 reblogged this · 8 years ago -
 letters-from-the-sky1 liked this · 8 years ago
letters-from-the-sky1 liked this · 8 years ago -
 nekoeka reblogged this · 8 years ago
nekoeka reblogged this · 8 years ago -
 kjvdh liked this · 8 years ago
kjvdh liked this · 8 years ago -
 thatcurlyhairedseason liked this · 8 years ago
thatcurlyhairedseason liked this · 8 years ago -
 planckwall reblogged this · 8 years ago
planckwall reblogged this · 8 years ago -
 mangosyfresas liked this · 8 years ago
mangosyfresas liked this · 8 years ago -
 krumpirka liked this · 8 years ago
krumpirka liked this · 8 years ago -
 jolly-to-the-sunset liked this · 8 years ago
jolly-to-the-sunset liked this · 8 years ago -
 alisonlovesbrains reblogged this · 8 years ago
alisonlovesbrains reblogged this · 8 years ago -
 gnyaa liked this · 8 years ago
gnyaa liked this · 8 years ago -
 forthesakeofbeinghuman liked this · 8 years ago
forthesakeofbeinghuman liked this · 8 years ago -
 darkmaster07 reblogged this · 8 years ago
darkmaster07 reblogged this · 8 years ago -
 darkmaster07 liked this · 8 years ago
darkmaster07 liked this · 8 years ago -
 bluewhales-and-pokemans liked this · 8 years ago
bluewhales-and-pokemans liked this · 8 years ago -
 leanna-lynn-little liked this · 8 years ago
leanna-lynn-little liked this · 8 years ago -
 parktrippin liked this · 8 years ago
parktrippin liked this · 8 years ago -
 lefthandrightbrain reblogged this · 8 years ago
lefthandrightbrain reblogged this · 8 years ago -
 donqu1x0te-blog reblogged this · 8 years ago
donqu1x0te-blog reblogged this · 8 years ago -
 donqu1x0te-blog liked this · 8 years ago
donqu1x0te-blog liked this · 8 years ago -
 queenofeire liked this · 8 years ago
queenofeire liked this · 8 years ago -
 blog-electricjello-blog liked this · 8 years ago
blog-electricjello-blog liked this · 8 years ago -
 savagesherbick liked this · 8 years ago
savagesherbick liked this · 8 years ago -
 xpandabeardontcarex reblogged this · 8 years ago
xpandabeardontcarex reblogged this · 8 years ago
A pharmacist and a little science sideblog. "Knowledge belongs to humanity, and is the torch which illuminates the world." - Louis Pasteur
215 posts








