Around A Year Ago, Scientists Determined The Structure Of The SARS-CoV-2 Spike Protein. Here’s A Look

Around a year ago, scientists determined the structure of the SARS-CoV-2 spike protein. Here’s a look at how it was done and how it helped the fight against #COVID19 in the latest edition of #ChemVsCOVID with the Royal Society of Chemistry: https://ift.tt/3pZiZe9 https://ift.tt/3002NPh
More Posts from Amateurchemstudent and Others


Follow @productive-tips for more tips and content like this posted daily! Handpicked and curated with love :)
Polarity, Resonance, and Electron Pushing: Crash Course Organic Chemistry #10:
We’ve all heard the phrase “opposites attract.” It may or may not be true for people, but it’s definitely true in organic chemistry. In this episode of Crash Course Organic Chemistry, we’re learning about electronegativity, polarity, resonance structures, and resonance hybrids. We’ll practice a very important skill for this course that will help us avoid a lot of memorization in the future: electron pushing. It’ll be a lot of trial and error at first, but we all start somewhere!
Alkanes: Saturated Hydrocarbons
So you want to be an organic chemist? Well, learning about hydrocarbons such as alkanes is a good place to start…
Alkanes are a homologous series of hydrocarbons, meaning that each of the series differs by -CH2 and that the compounds contain carbon and hydrogen atoms only. Carbon atoms in alkanes have four bonds which is the maximum a carbon atom can have - this is why the molecule is described to be saturated. Saturated hydrocarbons have only single bonds between the carbon atoms.
The general formula of an alkane is CnH2n+2 where n is the number of carbons. For example, if n = 3, the hydrocarbon formula would be C3H8 or propane. Naming alkanes comes from the number of carbons in the chain structure.
Here are the first three alkanes. Each one differs by -CH2.

Shorter chain alkanes are gases at room temperature, medium ones are liquids and the longer chain alkanes are waxy solids.
Alkanes have these physical properties:
1. They are non-polar due to the tiny difference in electronegativity between the carbon and hydrogen atoms.
2. Only Van der Waals intermolecular forces exist between alkane molecules. The strength of these increase as relative molecular mass increases therefore so does the melting/boiling point.
3. Branched chain alkanes have lower melting and boiling points than straight chain isomers with the same number of carbons. Since atoms are further apart due to a smaller surface area in contact with each other, the strength of the VDWs is decreased.
4. Alkanes are insoluble in water but can dissolve in non-polar liquids like hexane and cyclopentane. Mixtures are separated by fractional distillation or a separating funnel.
The fractional distillation of crude oil, cracking and the combustion equations of the alkanes will be in the next post.
SUMMARY
Alkanes are a homologous series of hydrocarbons. Carbon atoms in alkanes have four bonds which is the maximum a carbon atom can have - this is why the molecule is described to be saturated. Saturated hydrocarbons have only single bonds between the carbon atoms.
The general formula of an alkane is CnH2n+2 where n is the number of carbons.
Shorter chain alkanes are gases at room temperature, medium ones are liquids and the longer chain alkanes are waxy solids.
They are non-polar.
Only Van der Waals intermolecular forces exist between alkane molecules. The strength of these increase as relative molecular mass increases therefore so does the melting/boiling point.
Branched chain alkanes have lower melting and boiling points than straight chain isomers with the same number of carbons.
Alkanes are insoluble in water but can dissolve in non-polar liquids like hexane. Mixtures are separated by fractional distillation or a separating funnel.
Covalent and Dative Bonds
Covalent and dative (sometimes called co-ordinate) bonds occur between two or more non-metals, e.g. carbon dioxide, water, methane and even diamond. But what actually are they?
A covalent bond is a chemical bond that involves the sharing of electron pairs between atoms. They are found in molecular elements or compounds such as chlorine or sulfur, but also in macromolecular elements and compounds like SiO2 and graphite. Covalent bonds are also found in molecular ions such as NH4+ and HCO3-.
Single covalent bonds have just one shared pair of electrons. Regularly, each atom provides one unpaired electron (the amount of unpaired electrons is usually equal to the number of covalent bonds which can be made) in the bond. Double covalent bonds have two shared pairs of electrons, represented by a double line between atoms, for example, O=C=O (CO2). Triple covalent bonds can also occur such as those in N ≡ N.
Dot and cross diagrams represent the arrangement of electrons in covalently bonded molecules. A shared pair of electrons is represented by a dot and a cross to show that the electrons come from different atoms.
Unpaired electrons are used to form covalent bonds as previously mentioned. The unpaired electrons in orbitals of one atom can be shared with another unpaired electron in an orbital but sometimes atoms can promote electrons into unoccupied orbitals in the same energy level to form more bonds. This does not always occur, however, meaning different compounds can be formed - PCl3 and PCl4 are examples of this.

An example where promotion is used is in sulfur hexafluoride (SF6). The regular configuration of sulfur atoms is 1s2 2s2 2p6 3s2 3p4. It promotes, as shown in the diagram (see excited state), two electrons: one from the 3s electrons to the 3d orbital and one from the 3p to the 3d. Therefore there are 6 unpaired electrons for fluorine atoms to join. It has an octahedral structure.

An atom which has a lone pair (a pair of electrons uninvolved in bonding) of electrons can form a coordinate bond with the empty orbital of another atom. It essentially donates an electron into this orbital which when formed, acts the same as a normal covalent bond. A coordinate bond therefore contains a shared pair of electrons that have come from one atom.

When ammonia reacts with a H+ ion, a coordinate bond is formed between the lone pair on the ammonia molecule and the empty 1s sub-shell in the H+ ion. An arrow represents the dative covalent bond (coordinate bond). Charges on the final ion must be showed.
Summary
A covalent bond is a chemical bond that involves the sharing of electron pairs between atoms. They are found in molecular elements or compounds as well as in macromolecular elements and compounds. Also found in molecular ions.
Single covalent bonds have just one shared pair of electrons. Double covalent bonds have two shared pairs of electrons, represented by a double line between atoms. Triple covalent bonds can also occur.
Dot and cross diagrams represent the arrangement of electrons in covalently bonded molecules. A shared pair of electrons is represented by a dot and a cross to show that the electrons come from different atoms.
Unpaired electrons are used to form covalent bonds - they can be shared with another unpaired electron in an orbital but sometimes atoms can promote electrons into unoccupied orbitals in the same energy level to form more bonds. This does not always occur, however, meaning different compounds can be formed.
An example where promotion is used is in sulfur hexafluoride (SF6).
An atom which has a lone pair (a pair of electrons uninvolved in bonding) of electrons can form a coordinate bond with the empty orbital of another atom.
It donates an electron into this orbital which when formed, acts the same as a normal covalent bond. A coordinate bond therefore contains a shared pair of electrons that have come from one atom.
When ammonia reacts with a H+ ion, a coordinate bond is formed between the lone pair on the ammonia molecule and the empty 1s sub-shell in the H+ ion. An arrow represents the dative covalent bond (coordinate bond). Charges on the final ion must be showed.


Good news! You CAN rewire your brain. Through the same mechanism as forming habits, you can change your automatic thought patterns. When you catch yourself with unhealthy thoughts, STOP, and make a conscious effort to replace that thought pattern with a more beneficial/healthy thought pattern. Keep doing that whenever those thoughts occur. Slowly but surely your mind’s default voice will match what you’re teaching it.
Follow @productive-tips for more tips and content like this posted daily! Handpicked and curated with love :)
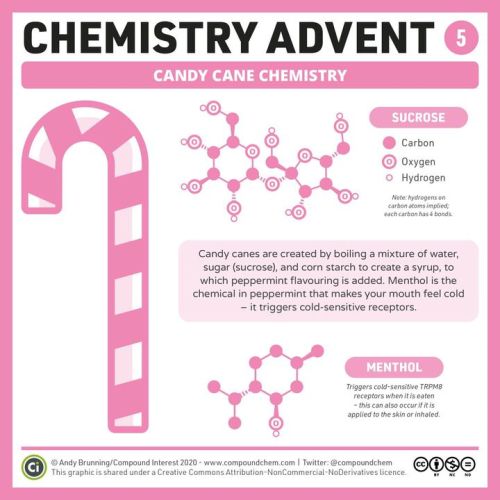
It’s day 5 of #ChemAdvent – here’s why peppermint candy canes make your mouth feel cold! bit.ly/chemadvent2020 https://ift.tt/2JM6bZ7
The Name’s Bond ... Ionic Bond.
This is the first in my short series of the three main types of bond - ionic, metallic and covalent. In this, you’ll learn about the properties of the compounds, which atoms they’re found between and how the bonds are formed. Enjoy!
When electrons are transferred from a metal to a non-metal, an ionic compound is formed. Metals usually lose electrons and non-metals usually gain them to get to a noble gas configuration. Transition metals do not always achieve this.
Charged particles that have either lost or gained electrons are called ions and are no longer neutral - metal atoms lose electrons to become positive ions (cations) whereas non-metals gain electrons to become negative ions (anions).
The formation of these ions is usually shown using electron configurations. Make sure you know that the transfer of electrons is not the bond but how the ions are formed.
An ionic bond is the electrostatic attraction between oppositely charged ions.
You need to know how to explain how atoms react with other atoms and for this the electron configurations are needed. You can use dot and cross diagrams for this.

Ionic solids hold ions in 3D structures called ionic lattices. A lattice is a repeating 3D pattern in a crystalline solid. For example, NaCl has a 6:6 arrangement - each Na+ ion is surrounded by 6 Cl- and vice versa.
Ionic solids have many strong electrostatic attractions between their ions. The crystalline shape can be decrepitated (cracked) on heating. Ionic Lattices have high melting and boiling points since they need more energy to break because atoms are held together by lots of strong electrostatic attractions between positive and negative ions. The boiling point of an ionic compound depends on the size of the atomic radius and the charge of the ion. The smaller the ion and the higher the charge, the stronger attraction.

Look at this diagram. It shows how atomic radius decreases across a period regularly. This is not the case with the ions. Positive ions are usually smaller than the atoms they came from because metal atoms lose electrons meaning the nuclear charge increases which draws the electrons closer to the nucleus. For negative ions, they become larger because repulsion between electrons moves them further away - nuclear charge also decreases as more electrons to the same number of protons.
Ionic substances can conduct electricity through the movement of charged particles when molten or dissolved (aqueous). This is because when they are like this, electrons are free to move and carry a charge. Ionic solids cannot conduct electricity.

Ionic compounds are usually soluble in water. This is because the polar water molecules cluster around ions which have broken off the lattice and so separate them from each other. Some substances like aluminium oxide have too strong electrostatic attractions so water cannot break up the lattice - it is insoluble in water.
Molecular ions such as sulfate, nitrate, ammonium or carbonate can exist within ionic compounds. These compounds may have covalent bonds within the ions but overall they are ionic and exhibit thee properties described above.
SUMMARY
When electrons are transferred from a metal to a non-metal, an ionic compound is formed.
Charged particles that have either lost or gained electrons are called ions and are no longer neutral - metal atoms lose electrons to become positive ions (cations) whereas non-metals gain electrons to become negative ions (anions).
The formation of these ions is usually shown using electron configurations. The transfer of electrons is not the bond but how the ions are formed.
An ionic bond is the electrostatic attraction between oppositely charged ions.
Ionic solids hold ions in 3D structures called ionic lattices. A lattice is a repeating 3D pattern in a crystalline solid.
Ionic solids have many strong electrostatic attractions between their ions. The crystalline shape can be decrepitated (cracked) on heating.
Ionic Lattices have high melting and boiling points since they need more energy to break because atoms are held together by lots of strong electrostatic attractions between positive and negative ions.
The boiling point of an ionic compound depends on the size of the atomic radius and the charge of the ion. The smaller the ion and the higher the charge, the stronger attraction.
Positive ions are usually smaller than the atoms they came from because metal atoms lose electrons meaning the nuclear charge increases which draws the electrons closer to the nucleus. Negative ions become larger because repulsion between electrons moves them further away - nuclear charge also decreases as more electrons to the same number of protons.
Ionic substances can conduct electricity through the movement of charged particles when molten or dissolved (aqueous). This is because when they are like this, electrons are free to move and carry a charge. Ionic solids cannot conduct electricity.
Ionic compounds are usually soluble in water because the polar water molecules cluster around ions which have broken off the lattice and so separate them from each other.
Some substances like aluminium oxide have too strong electrostatic attractions so water cannot break up the lattice - it is insoluble in water.
Molecular ions such as sulfate, nitrate, ammonium or carbonate can exist within ionic compounds. These compounds may have covalent bonds within the ions but overall they are ionic and exhibit thee properties described above.

#Thunderstorms are hitting the UK this week – here’s how thunder and lightning happen and some of the chemistry going on during a storm: https://ift.tt/2XUCKZc https://ift.tt/3gJ7ALD
-
 carljparker liked this · 3 years ago
carljparker liked this · 3 years ago -
 1biomed reblogged this · 3 years ago
1biomed reblogged this · 3 years ago -
 pleasurehunter2000 liked this · 4 years ago
pleasurehunter2000 liked this · 4 years ago -
 eternalodetoathena reblogged this · 4 years ago
eternalodetoathena reblogged this · 4 years ago -
 anxiolytica reblogged this · 4 years ago
anxiolytica reblogged this · 4 years ago -
 chatelaine-panda liked this · 4 years ago
chatelaine-panda liked this · 4 years ago -
 quacktangent liked this · 4 years ago
quacktangent liked this · 4 years ago -
 annebanan liked this · 4 years ago
annebanan liked this · 4 years ago -
 lycragym85 liked this · 4 years ago
lycragym85 liked this · 4 years ago -
 zacolyn reblogged this · 4 years ago
zacolyn reblogged this · 4 years ago -
 bug-catcher-jecht liked this · 4 years ago
bug-catcher-jecht liked this · 4 years ago -
 inner-space-oddity liked this · 4 years ago
inner-space-oddity liked this · 4 years ago -
 stickitoutorstartagain liked this · 4 years ago
stickitoutorstartagain liked this · 4 years ago -
 perpetually-attempting-man liked this · 4 years ago
perpetually-attempting-man liked this · 4 years ago -
 symbiotic-science reblogged this · 4 years ago
symbiotic-science reblogged this · 4 years ago -
 amateurchemstudent reblogged this · 4 years ago
amateurchemstudent reblogged this · 4 years ago -
 amateurchemstudent liked this · 4 years ago
amateurchemstudent liked this · 4 years ago -
 mintschlar reblogged this · 4 years ago
mintschlar reblogged this · 4 years ago -
 jwacademy reblogged this · 4 years ago
jwacademy reblogged this · 4 years ago -
 satyasuman liked this · 4 years ago
satyasuman liked this · 4 years ago -
 arecipeforfeels liked this · 4 years ago
arecipeforfeels liked this · 4 years ago -
 friednickelbagelkid liked this · 4 years ago
friednickelbagelkid liked this · 4 years ago -
 homosapien-cupcake reblogged this · 4 years ago
homosapien-cupcake reblogged this · 4 years ago -
 homosapien-cupcake liked this · 4 years ago
homosapien-cupcake liked this · 4 years ago -
 jairmboy liked this · 4 years ago
jairmboy liked this · 4 years ago -
 vie-belle-vie liked this · 4 years ago
vie-belle-vie liked this · 4 years ago -
 newmuse liked this · 4 years ago
newmuse liked this · 4 years ago -
 ophis969 liked this · 4 years ago
ophis969 liked this · 4 years ago -
 inodiate liked this · 4 years ago
inodiate liked this · 4 years ago -
 chemicalmage liked this · 4 years ago
chemicalmage liked this · 4 years ago -
 floragraysteel liked this · 4 years ago
floragraysteel liked this · 4 years ago -
 sakuraluck liked this · 4 years ago
sakuraluck liked this · 4 years ago -
 garazb301-blogspot-com liked this · 4 years ago
garazb301-blogspot-com liked this · 4 years ago -
 titttiessss liked this · 4 years ago
titttiessss liked this · 4 years ago -
 honeyharmony liked this · 4 years ago
honeyharmony liked this · 4 years ago -
 yngchwita liked this · 4 years ago
yngchwita liked this · 4 years ago -
 tanvirranarabbi liked this · 4 years ago
tanvirranarabbi liked this · 4 years ago -
 kramit-the-forg liked this · 4 years ago
kramit-the-forg liked this · 4 years ago -
 graspurt liked this · 4 years ago
graspurt liked this · 4 years ago -
 satans-desire liked this · 4 years ago
satans-desire liked this · 4 years ago


