Unusual Fluid Behavior Observed In Microgravity

Unusual fluid behavior observed in microgravity
Normally when a liquid is heated above its boiling point, it evaporates, turning into a vapor. But when scientists recently performed an experiment on the International Space Station (ISS), they observed that the vapor near a heat pipe condensed into a liquid even when the temperature was 160 K above the substance’s normal boiling point. The results show that microgravity significantly alters the processes of evaporation and condensation, but the scientists do not yet have a complete explanation for the phenomenon.
The research team, consisting of scientists from Rensselaer Polytechnic Institute and the NASA Glenn Research Center, have published a paper on the surprising observations in a recent issue of Physical Review Letters.
This is not the first time that unexpected behavior in heat pipes, which are devices used to cool components of a spacecraft, has been observed in microgravity. In 2015, many of the same researchers made a related, counterintuitive observation during experiments conducted on the ISS.
At that time, the researchers observed that increasing the heat input to a heat pipe did not cause the device to dry out near the heated end as it does on Earth, but instead it caused liquid accumulation there. At the time, the processes responsible for this phenomenon were not completely understood.
Read more.
More Posts from Hannahhaifisch and Others

cube

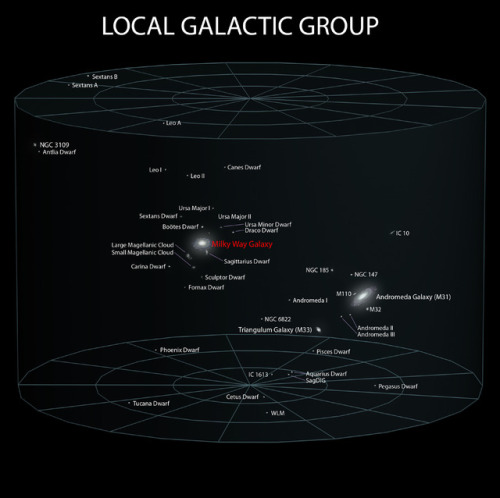
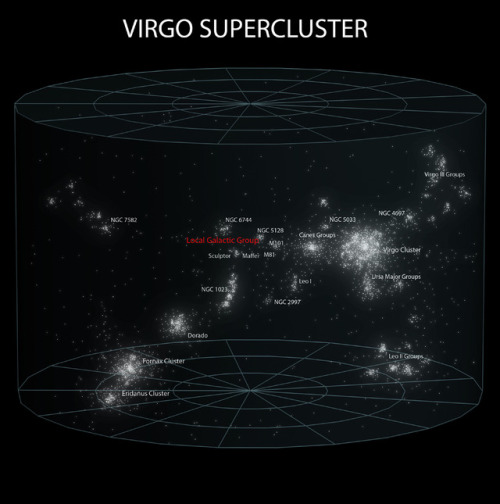
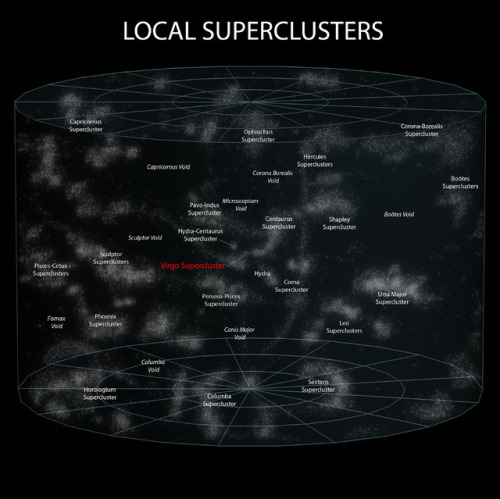
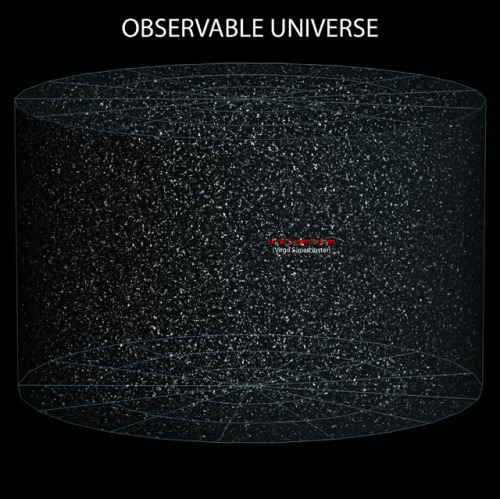
~ wikimedia commons

Robert Irwin @ Art Basel 2015
Black 3, 2008
via







TRAPPIST-1 Planets - Flyaround Animation
Credit: NASA/Spitzer

Hexagons and rhombis spreading out

Sentinels of the Arctic http://go.nasa.gov/2n1ynuo

Mutation by Ben Butler 30″ x 32″ x 16″, cedar, 2006
Heartwood on Tumblr


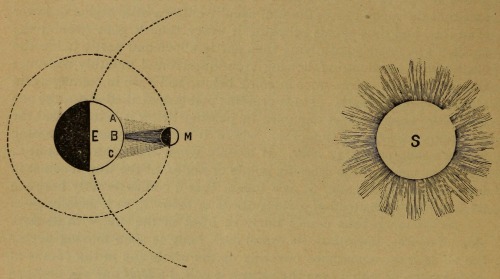
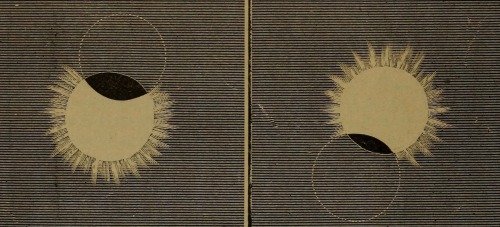



Samuel W. Hall. Corona of an Eclipse, Moon Hiding Lower and Upper Limb of Sun, Eclipse of the Sun, Moon Hiding Upper and Lower Limb of Sun, Comparative Size of the Planets, Size of the Sun, Elliptical Orbit. Sunshine and Moonlight; with; also, a Flash of Comets, Meteors and Shooting Stars, and a Twinkle of Starlight. 1889. Contd from here
In this short film, the Macro Room team plays with the diffusion of ink in water and its interaction with various shapes. Injecting ink with a syringe results in a beautiful, billowing turbulent plume. By fiddling with the playback time, the video really highlights some of the neat instabilities the ink goes through before it mixes. Note how the yellow ink at 1:12 breaks into jellyfish-like shapes with tentacles that sprout more ink; that’s a classic form of the Rayleigh-Taylor instability, driven by the higher density ink sinking through the lower density water. Ink’s higher density is what drives the ink-falls flowing down the flowers in the final segment, too. Definitely take a couple minutes to watch the full video. (Image and video credit: Macro Room; via James H./Flow Vis)




Substances don’t have to be a liquid or a gas to behave like a fluid. Swarms of fire ants display viscoelastic properties, meaning they can act like both a liquid and a solid. Like a spring, a ball of fire ants is elastic, bouncing back after being squished (top image). But the group can also act like a viscous liquid. A ball of ants can flow and diffuse outward (middle image). The ants are excellent at linking with one another, which allows them to survive floods by forming rafts and to escape containers by building towers.
Researchers found the key characteristic is that ants will only maintain links with nearby ants as long as they themselves experience no more than 3 times their own weight in load. In practice, the ants can easily withstand 100 times that load without injury, but that lower threshold describes the transition point between ants as a solid and ants as a fluid. If an ant in a structure is loaded with more force, he’ll let go of his neighbors and start moving around.
When they’re linked, the fire ants are close enough together to be water-repellent. Even if an ant raft gets submerged (bottom image), the space between ants is small enough that water can’t get in and the air around them can’t get out. This coats the submerged ants in their own little bubble, which the ants use to breathe while they float out a flood. For more, check out the video below and the full (fun and readable!) research paper linked in the credits. (Video and image credits: Vox/Georgia Tech; research credit: S. Phonekeo et al., pdf; submitted by Joyce S., Rebecca S., and possibly others)
-
 sciencenerd4-blog liked this · 8 years ago
sciencenerd4-blog liked this · 8 years ago -
 thegamejustgotmorefierce reblogged this · 8 years ago
thegamejustgotmorefierce reblogged this · 8 years ago -
 noelong-blog liked this · 8 years ago
noelong-blog liked this · 8 years ago -
 rovafbeats liked this · 8 years ago
rovafbeats liked this · 8 years ago -
 rametarin liked this · 8 years ago
rametarin liked this · 8 years ago -
 mycarandmyguitar liked this · 8 years ago
mycarandmyguitar liked this · 8 years ago -
 hannahhaifisch reblogged this · 8 years ago
hannahhaifisch reblogged this · 8 years ago -
 hannahhaifisch liked this · 8 years ago
hannahhaifisch liked this · 8 years ago -
 superjaybird liked this · 8 years ago
superjaybird liked this · 8 years ago -
 illegal-driftwood liked this · 8 years ago
illegal-driftwood liked this · 8 years ago -
 kastronautt liked this · 8 years ago
kastronautt liked this · 8 years ago -
 edges-of-empires liked this · 8 years ago
edges-of-empires liked this · 8 years ago -
 cydi liked this · 8 years ago
cydi liked this · 8 years ago -
 andygoestohell liked this · 8 years ago
andygoestohell liked this · 8 years ago -
 charlesh55 liked this · 8 years ago
charlesh55 liked this · 8 years ago -
 tbdmsdmlm liked this · 8 years ago
tbdmsdmlm liked this · 8 years ago -
 fingersintheshapeofguns liked this · 8 years ago
fingersintheshapeofguns liked this · 8 years ago -
 poets-in-a-teenage-age reblogged this · 8 years ago
poets-in-a-teenage-age reblogged this · 8 years ago -
 poets-in-a-teenage-age liked this · 8 years ago
poets-in-a-teenage-age liked this · 8 years ago -
 materialsscienceandengineering reblogged this · 8 years ago
materialsscienceandengineering reblogged this · 8 years ago